- Department of Neurosurgery, Kansai Rosai Hospital, Amagasaki, Hyogo, Japan.
Correspondence Address:
Shingo Toyota, Department of Neurosurgery, Kansai Rosai Hospital, Amagasaki, Hyogo, Japan.
DOI:10.25259/SNI_749_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Tomoaki Murakami, Shingo Toyota, Takuya Suematsu, Yuki Wada, Takeshi Shimizu, Takuyu Taki. Carotid–carotid crossover bypass after mechanical thrombectomy for internal carotid artery occlusion due to plaque from stenosed innominate artery. 30-Sep-2021;12:480
How to cite this URL: Tomoaki Murakami, Shingo Toyota, Takuya Suematsu, Yuki Wada, Takeshi Shimizu, Takuyu Taki. Carotid–carotid crossover bypass after mechanical thrombectomy for internal carotid artery occlusion due to plaque from stenosed innominate artery. 30-Sep-2021;12:480. Available from: https://surgicalneurologyint.com/surgicalint-articles/11148/
Abstract
Background: The treatment for internal carotid artery occlusion (ICAO) due to innominate artery stenosis is not well established. We herein describe a case of carotid–carotid crossover bypass and common carotid artery (CCA) ligation after mechanical thrombectomy for ICAO due to a plaque from the stenosed innominate artery.
Case Description: A 70-year-old man was transferred to our hospital because of left-sided hemiparalysis. Head magnetic resonance imaging/angiography showed a cerebral infarction in the right middle cerebral artery area and the right ICAO due to a plaque from the stenosed innominate artery. Immediately, we performed mechanical thrombectomy and successfully attained partial revascularization (Thrombolysis in Cerebral Infarction Grade 2B). After a conference with cardiovascular group, we performed carotid–carotid crossover bypass and the right CCA ligation. The treatment was successful, and no complications occurred.
Conclusion: Carotid–carotid crossover bypass and CCA ligation may be a better option for innominate artery stenosis in selected patients.
Keywords: Carotid–carotid crossover bypass, Cerebral infarction, Innominate artery stenosis, Tandem, Thrombectomy
INTRODUCTION
Atherosclerotic stenosis of the innominate artery (IA) is reportedly rare, accounting for 0.5– 2.5% of all vascular lesions.[
We herein describe carotid–carotid crossover bypass and common carotid artery (CCA) ligation in a patient with symptomatic IAS after mechanical thrombectomy for ICA occlusion (ICAO) due to a plaque from the stenosed IA. To the best of our knowledge, no such case has been reported to date.
CASE DESCRIPTION
A 70-year-old man was admitted to our hospital with left hemiparalysis and a National Institutes of Health Stroke Scale score of 8. He had been diagnosed with subtle right cerebral infarction [
Figure 1:
Pretreatment imaging (a and b) 9 months before presentation and (c, d) on admission before thrombectomy. (a) DWI revealed subtle right cerebral infarction indicating artery to artery embolism. (b) IA angiogram showed right IA–SA–proximal CCA stenosis. (c) Right ICA was occluded on MRA. (d) DWI showed acute cerebral infarction in the right middle cerebral artery area (DWI–Alberta Stroke Program Early Computed Tomography Score of 9).
Mechanical thrombectomy
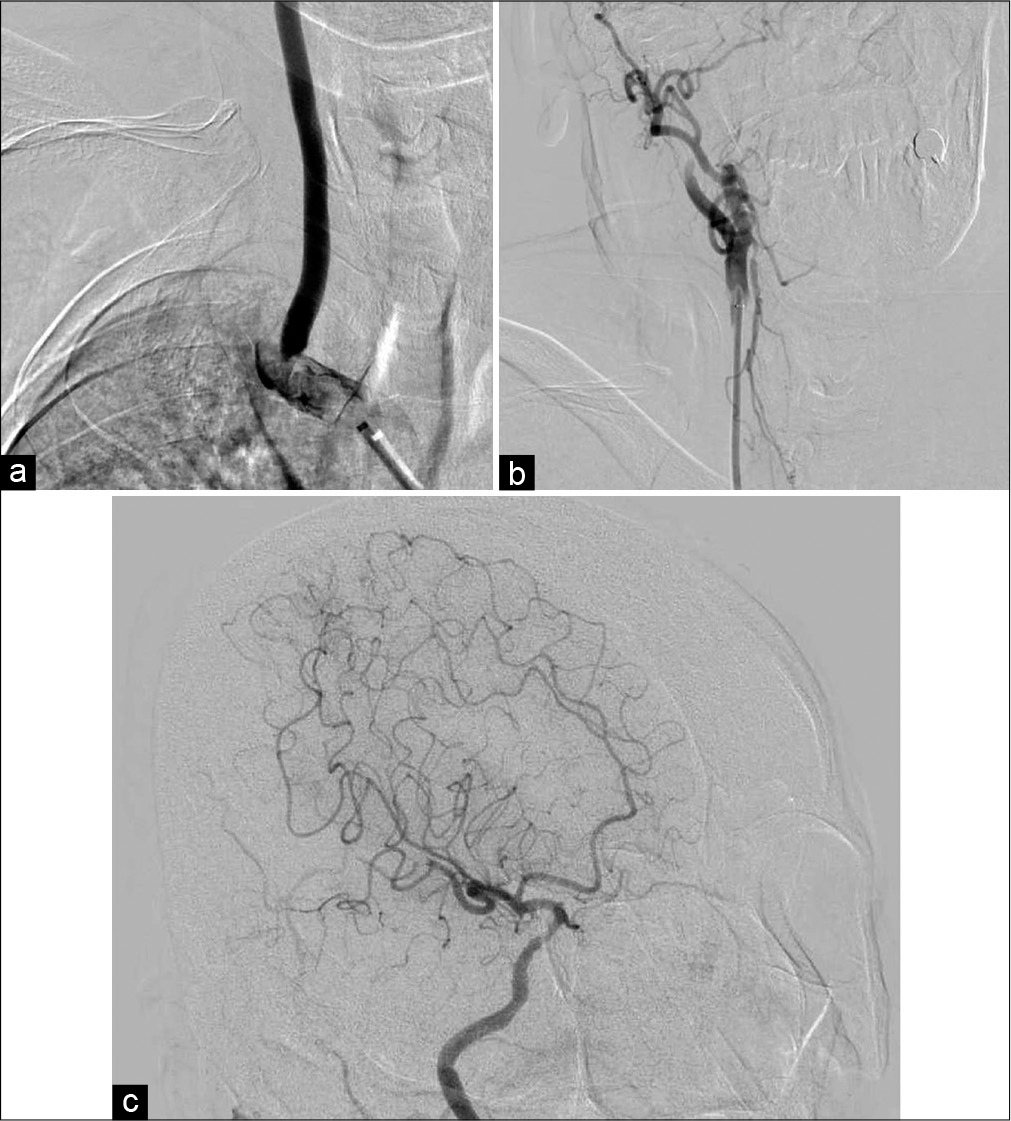
Because the duration since symptom onset exceeded 4.5 h, the patient was not a candidate for intravenous injection of tissue plasminogen activator. The endovascular procedure was started through a 9-Fr femoral sheath under local anesthesia. A 9-Fr balloon guiding catheter (Optimo; Tokai Medical Products, Aichi, Japan) was placed in the IA. Angiography showed severe stenosis from the IA to the proximal CCA and occlusion of the right SA [
Figure 2:
Diagnostic angiogram and mechanical thrombectomy. (a) Frontal view of IA angiogram showed severe stenosis from the IA to proximal CCA and occlusion of the right SA. (b) Frontal view of right CCA angiogram revealed occlusion of the right ICA. (c) Oblique view of right ICA angiogram showed partial recanalization (Thrombolysis in Cerebral Infarction grade 2b) without several branches.
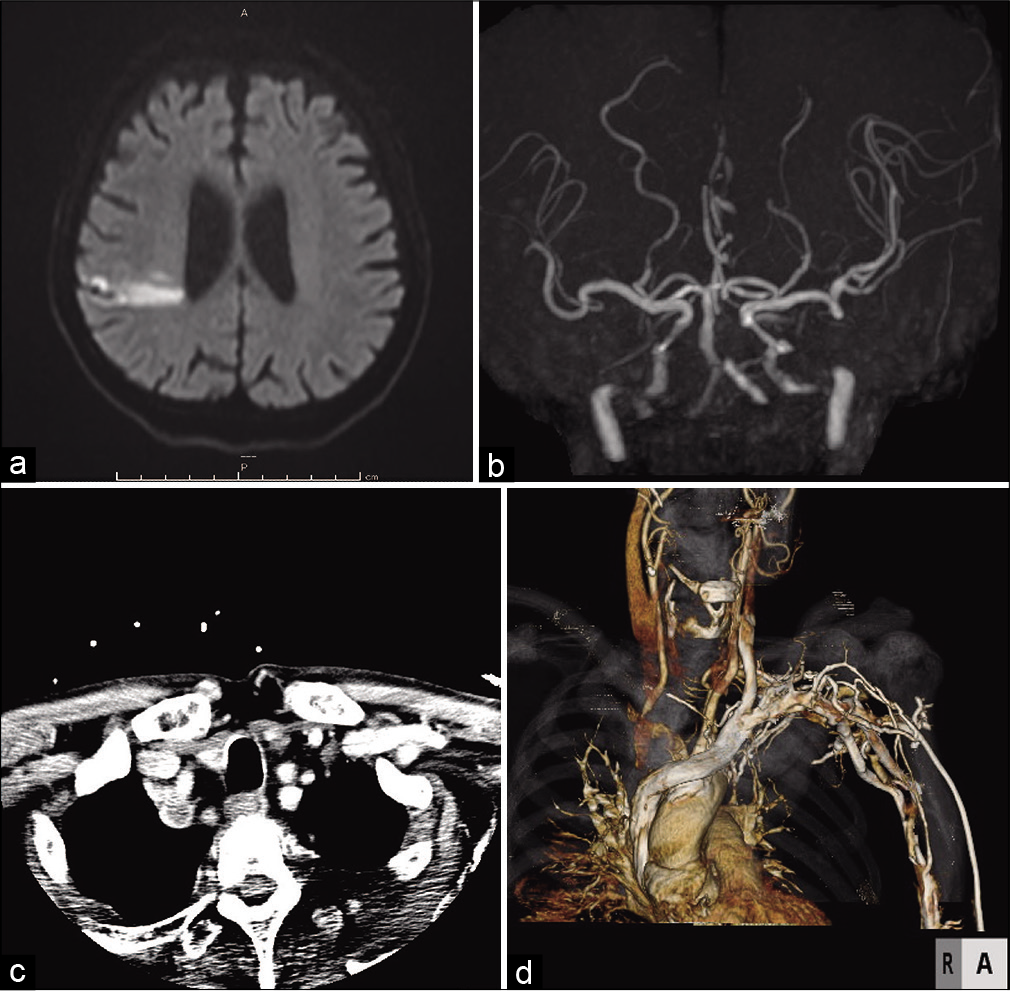
Figure 3:
Post thrombectomy head DWI and neck CTA. (a) DWI showed localized ischemic change in the territory of the right MCA that was almost identical to the preoperative DWI. (b) MRA showed recanalization of the right ICA and MCA without several distal branches. (c) The axial view of neck CTA indicated a massive plaque occupying the IA and right SA (d) CTA showed severe stenosis of the IA and proximal CCA as well as occlusion of the right SA.
Carotid–carotid crossover bypass and CCA ligation
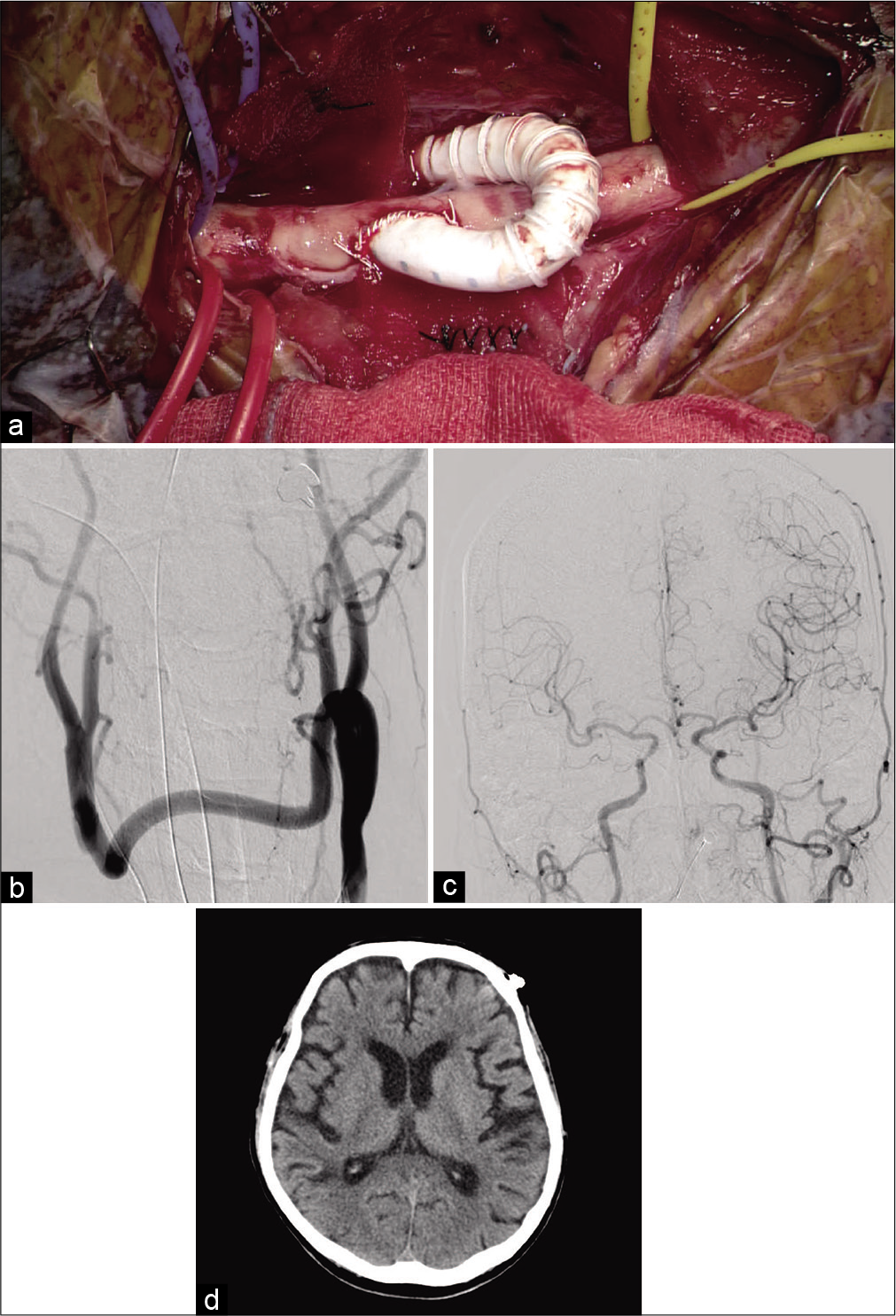
On the 35th hospital day, we performed carotid–carotid crossover bypass and CCA ligation to prevent recurrent embolization from the stenosed IA. The surgery was performed in a hybrid operating room. Endotracheal intubation anesthesia was provided, and the patient’s neck was stretched in the supine position. Monitoring was performed using near infrared spectroscopy (INVOS system; Medtronic, Minneapolis, MN, USA). Linear skin incisions of 6 cm were made bilaterally along the anterior edges of the sternocleidomastoid. The bilateral CCA, ICA, and external carotid artery were exposed; the retropharyngeal space was explored with the fingers; and a 6-mm heparin-bound polytetrafluoroethylene (PTFE) vascular graft (Propaten; W. L. Gore and Associates, Inc. Newark, DE, USA) was crossed through the space. The graft was trimmed according to the bypass requirements. Before clamping the CCA for the anastomosis, 4000 U of systemic heparin was administered, and the patient’s systolic blood pressure increased by 20% over the basal value. First, end-to-side anastomosis between the graft and left CCA was performed with continuous suturing using CV-6 Gore-Tex (W. L. Gore and Associates, Inc.). The same procedure was then performed for the right CCA [
Figure 4:
(a) Microsurgical view and (b-d) postoperative imaging. (a) End-to-side anastomosis between the PTFE vascular graft through the retropharyngeal tunnel and the right CCA was performed. (b) Frontal view of left CCA angiogram revealed patency of the bypass and complete ligation of the right CCA. (c) Frontal view of left CCA angiogram revealed anterograde flow of the bilateral intracranial ICAs. (d) Postoperative computed tomography showed no remarkable change compared with preoperative computed tomography.
Postoperative therapy was performed in accordance with the usual postoperative management of carotid endarterectomy. We started antiplatelet therapy (clopidogrel 75 mg/day) for lifetime. And the cervical collar was used to protect the bleeding from surgical wound for three days.
The patient was discharged to home with no procedural complications (modified Rankin scale score of 1) on day 56.
DISCUSSION
To the best of our knowledge, this is the first report of carotid– carotid crossover bypass in a patient with symptomatic IAS after mechanical thrombectomy for ipsilateral ICAO. The optimal strategy for treating tandem proximal artery stenosis and ipsilateral distal artery occlusion is controversial.[
The treatment of IAS consists of a transthoracic approach, an extrathoracic approach, and an endovascular approach in some literature review.[
In the present case, we discussed optimal treatment for this IAS, including carotid–carotid crossover bypass and CCA ligation, SA–carotid bypass, IA - CCA bypass and endovascular surgery, with cardiac surgeons and cardiologists. SA–carotid bypass was not recommended because the occluded right SA was not suitable as a donor artery in the present case, and IA - CCA bypass which needed on-pump arrest procedure was invasive. Furthermore, in the present case, we considered that the plaque in the complex saddle lesion with SA occlusion was clinically unstable because cerebral infarction had already occurred twice in the ipsilateral lesion. After a conference with cardiovascular group, we selected carotid–carotid crossover bypass and CCA ligation as an elective surgery to treat the symptomatic IAS.
The previous studies have shown that carotid–carotid crossover bypass is effective and safe for the IA as well as for CCA occlusive and aneurysmal diseases.[
The materials currently used as vascular grafts for carotid– carotid crossover bypass are prosthetic vascular grafts[
On the other hands, it has been reported that some complications relating carotid-carotid crossover bypass such as bleeding (4%), stroke (4%), and early occlusion (4%) could occur.[
CONCLUSION
Even though the indication is limited, carotid–carotid crossover bypass and CCA ligation can be an effective and safe option for treatment of symptomatic IAS.
Authors’ contributions
Drs. Toyota, Taki, and Murakami contributed to the conception and design of the study. Drs. Toyota, Suematsu, Wada, Shimizu, Taki, and Murakami performed the surgery. Drs. Toyota, Taki, and Murakami contributed to the writing and revising of the manuscript.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Authors’ contributions
Drs. Toyota, Taki, and Murakami contributed to the conception and design of the study. Drs. Toyota, Suematsu, Wada, Shimizu, Taki, and Murakami performed the surgery. Drs. Toyota, Taki, and Murakami contributed to the writing and revising of the manuscript.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We thank Angela Morben, DVM, ELS, from Edanz Group ( https://en-author-services.edanz.com/ac), for editing a draft of this manuscript.
References
1. Akpinar CK, Gürkaş E, Aytac E. Carotid angioplasty-assisted mechanical thrombectomy without urgent stenting may be a better option in acute tandem occlusions. Interv Neuroradiol. 2017. 23: 405-11
2. Berguer R, Gonzalez JA. Revascularization by the retropharyngeal route for extensive disease of the extracranial arteries. J Vasc Surg. 1994. 19: 217-24
3. Berguer R, Morasch MD, Kline RA, Kazmers A, Friedland MS. Cervical reconstruction of the supra-aortic trunks: A 16-year experience. J Vasc Surg. 1999. 29: 239-46
4. Berguer R, Morasch MD, Kline RA. Transthoracic repair of innominate and common carotid artery disease: Immediate and long-term outcome for 100 consecutive surgical reconstructions. J Vasc Surg. 1998. 27: 34-41
5. Berguer R. The short retropharyngeal route for arterial bypass across the neck. Ann Vasc Surg. 1986. 1: 127-9
6. Brusett KA, Kwasnik EM, Marjani MA. Innominate artery saddle embolus: A pitfall for retrograde brachial embolectomy. J Vasc Surg. 1997. 25: 569-71
7. Chen C, Ye Z, Luo L, Guo Y, Chang Y, Ning X. Carotidcarotid artery crossover bypass with a synthetic vascular graft for symptomatic Type 1A common carotid artery occlusion. World Neurosurg. 2018. 111: e286-93
8. Klonaris C, Kouvelos GN, Kafeza M, Koutsoumpelis A, Katsargyris A, Tsigris C. Common carotid artery occlusion treatment: Revealing a gap in the current guidelines. Eur J Vasc Endovasc Surg. 2013. 46: 291-8
9. Kovacevic P, Velicki L, Ivanovic V, Kieffer E. Carotidcarotid bypass as an option in the treatment of infected pseudoaneurysm after prosthetic carotid replacement. Ann Vasc Surg. 2013. 27: 239.e13-6
10. Li ZY, Chen C, Ling C, He HY, Luo L, Li H. Surgical procedures including carotid-carotid crossover bypass and ring-stripping hybrid operation for rile’s Type 1A common carotid artery occlusion: An experience of 6 cases. Ann Transl Med. 2020. 8: 439
11. Morofuji Y, Matsunaga Y, Izumo T. Carotid-carotid bypass for a carotid artery aneurysm. World Neurosurg. 2020. 138: 7-8
12. Moustafa RR, Antoun NM, Coulden RA, Warburton EA, Baron JC. Stroke attributable to a calcific embolus from the brachiocephalic trunk. Stroke. 2006. 37: e6-8
13. Mpotsaris A, Bussmeyer M, Buchner H, Weber W. Clinical outcome of neurointerventional emergency treatment of extra-or intracranial tandem occlusions in acute major stroke: Antegrade approach with wallstent and solitaire stent retriever. Clin Neuroradiol. 2013. 23: 207-15
14. Naylor AR, Ricco JB, de Borst GJ, Debus S, de Haro J, Halliday A.editors. Editor’s choice-management of atherosclerotic carotid and vertebral artery disease: 2017 clinical practice guidelines of the European society for vascular surgery (ESVS). Eur J Vasc Endovasc Surg. 2018. 55: 3-81
15. Oweida SW, Ku DN, Justicz AG, Burnson B, Salam AA. Hemodynamic consequences of carotid-carotid bypass for innominate artery stenosis. J Vasc Surg. 1991. 13: 416-22
16. Ozsvath KJ, Roddy SP, Darling RC, Byrne J, Kreienberg PB, Choi D. Carotid-carotid crossover bypass: Is it a durable procedure?. J Vasc Surg. 2003. 37: 582-5
17. Paukovits TM, Lukács L, Bérczi V, Hirschberg K, Nemes B, Hüttl K. Percutaneous endovascular treatment of innominate artery lesions: A single-centre experience on 77 lesions. Eur J Vasc Endovasc Surg. 2010. 40: 35-43
18. van de Weijer MA, Vonken EJ, de Vries JP, Moll FL, Vos JA, de Borst GJ. Technical and clinical success and long-term durability of endovascular treatment for atherosclerotic aortic arch branch origin obstruction: Evaluation of 144 procedures. Eur J Vasc Endovasc Surg. 2015. 50: 13-20
19. Wilson MP, Murad MH, Krings T, Pereira VM, O’Kelly C, Rempel J. Management of tandem occlusions in acute ischemic stroke-intracranial versus extracranial first and extracranial stenting versus angioplasty alone: A systematic review and meta-analysis. J Neurointerv Surg. 2018. 10: 721-8
20. Yoneyama F, Sato F, Tokunaga C, Sakamoto H, Enomoto Y, Watanabe Y. Postoperative dysphagia in debranching thoracic endovascular aortic repair with retroesophageal carotid-carotid bypass. Ann Vasc Surg. 2017. 43: 315.e1-4