- Department of Neurosurgery, Emory University School of Medicine, Atlanta, Georgia, United States.
- Department of Pathology, Emory University School of Medicine, Atlanta, Georgia, United States.
Correspondence Address:
Gustavo Pradilla
Department of Neurosurgery, Emory University School of Medicine, Atlanta, Georgia, United States.
DOI:10.25259/SNI_770_2020
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Anil K. Roy1, Nefize Turan1, Pasang Wangmo1, Louis Nkrumah1, Stewart G. Neill2, Gustavo Pradilla1. Comparatıve assessment of thermal ınjury ınduced by bıpolar electrocautery systems ın a porcıne model. 08-Apr-2021;12:146
How to cite this URL: Anil K. Roy1, Nefize Turan1, Pasang Wangmo1, Louis Nkrumah1, Stewart G. Neill2, Gustavo Pradilla1. Comparatıve assessment of thermal ınjury ınduced by bıpolar electrocautery systems ın a porcıne model. 08-Apr-2021;12:146. Available from: https://surgicalneurologyint.com/surgicalint-articles/10697/
Abstract
Background: Bipolar electrocautery systems used during neurosurgical procedures have been shown to induce thermal injury to surrounding tissue. The goal of this study was to compare the thermal injury induced by two different systems commonly used in neurosurgical procedures (Silverglide by Stryker Corporation and SpetzlerMalis by Codman Neuro), with that of a newly introduced device (TRIOwand by NICO Corporation).
Methods: A farm swine underwent craniectomy and durotomy with subsequent exposure of cortical brain tissue. Electrocoagulation for the duration of 3 s was conducted with three different bipolar systems under comparable power settings. The maximal depth of thermal injury and mean area of injury in Hematoxylin and Eosin stained slides were quantified using Image J. The tissues were evaluated for vacuolization and ischemic damage. One-way ANOVA followed by post hoc Tukey test was utilized for statistical analysis. Alpha level was set at 0.05.
Results: TRIOwand lesions showed less depth of injury when compared to both Spetzler-Malis (P P = 0.048). Silverglide lesions showed significantly less depth of injury when compared to SpetzlerMalis lesions (P P P
Conclusion: Thermal damage is induced to varying extents by all bipolar systems. In this porcine model and under the conditions tested, bipolar cauterization with the TRIOwand resulted in less depth and decreased mean area of injury. Further studies are needed to characterize the injury caused by different bipolar systems with other settings and under surgical conditions in humans.
Keywords: Bipolar, Electrocautery, Porcine, Thermal damage,
INTRODUCTION
Bipolar electrocoagulation is a basic technique of modern neurosurgery and has undergone significant technological improvements since its introduction in 1940 by James Greenwood.[
Several techniques, including the use of active heat transfer and continuous irrigation have been introduced to minimize heat transfer away from the site of electrocoagulation along with minimizing charring and tissue adherence.[
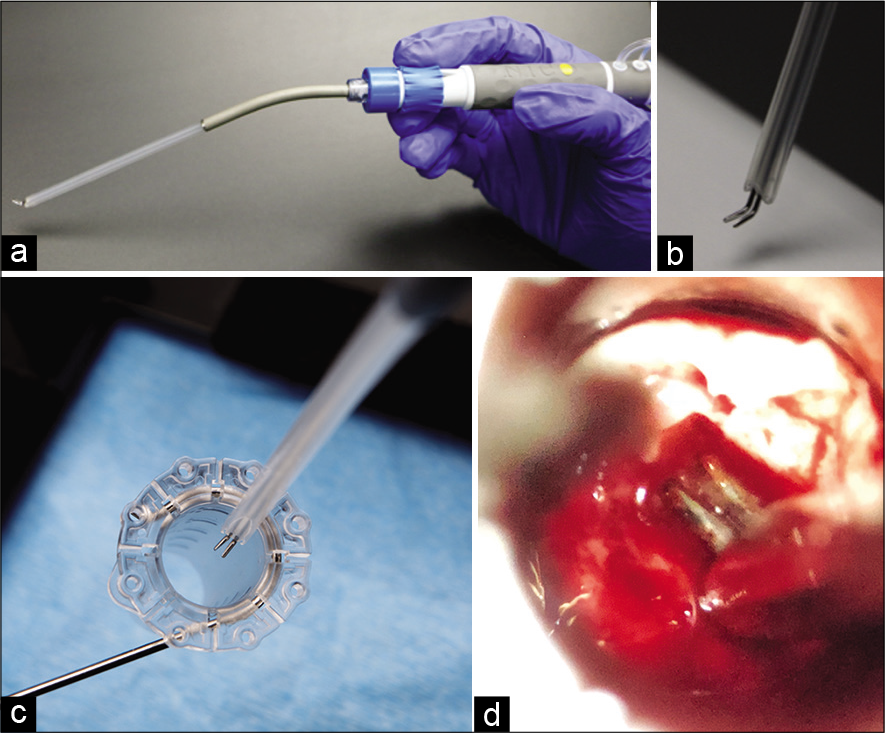
The TRIOwand bipolar cautery system (NICO Corporation, Indianapolis, Indiana) was recently introduced for working in narrow and deep surgical corridors that require minimal access ports for treatment of lesions located in the subcortical white matter or the cerebral ventricles [
METHODS
Experimental design
Bipolar electrocautery is primarily employed for hemostasis during active bleeding, which is difficult to accurately replicate in experimental settings. Repeated measures in the same subject are required as physiological animal conditions such as blood pressure, rheology, vessel diameter, and others can vary significantly among subjects despite standardized anesthetic and surgical techniques. Thus our study focused on assessment of tissue damage due to heat transfer for a fixed duration of time under nonhemorrhagic conditions in a single subject. The frontal and parietal cortical surface of a swine was divided into three separate regions for electrocoagulation utilizing three different bipolar systems. Region 1 was cauterized using the Spetzler-Malis bipolar with a Codman generator set at 25 “Malis Units.” Region 2 was cauterized using the TRIOwand with a Valley Lab generator set at 4 watts. Region 3 was electrocoagulated using the Silverglide with a Valley Lab generator set at 4 watts. The Spetzler-Malis generator manual provides a manufacturer generated equivalency from the “Malis units” to the standard Watt output in the Valley lab generator and these settings were tested in the soft tissues of the animal before cortical brain cauterization for macroscopic cauterization equivalency as subjectively estimated by the neurosurgeon. Within each region, seven separate lesions were made, with each lesion requiring active cauterization for 3 s. The lesions were made without the use of irrigation to assess for maximal possible injury for the duration of electrocoagulation. The VITOM HD II Exoscope (Karl Storz, El Segundo, CA) was used for image acquisition and video documentation. All animal procedures were approved by T3 Labs Institutional Animal Care and Use Committee. All experimental studies comply with animal care complied with the Guide for the Care and Use of Laboratory Animals. All bipolar systems tested have received FDA approval for clinical human use.
Surgical technique
A farm swine greater than 30 kg was utilized in the study, and the surgery was performed in a dedicated surgical animal facility (T3 Labs, Atlanta, GA). The animal was placed in a ventral recumbent position and administered flunixin 2.0 mg/kg IM and atropine 0.05 mg/kg IM for preoperative management. Anesthesia was subsequently induced using ketamine 20 mg/kg with xylazine 2.0 mg/kg IM and maintained using 0.5–5% isofluorane (with nitrous oxide) for the duration of the surgical procedure. A longitudinal midline skin incision was made in the dorsal head. The scalp was retracted and large bilateral craniectomies were completed with a high speed drill, curettes and Kerrison rongeurs. The dura was subsequently opened and three separate cortical regions were identified with lesions performed as described earlier [
Figure 2:
Cranial exposure of porcine brain. (a and b) Gross anatomical appearance before and after electrocautery with TRIOwand (c and d) Gross anatomical images before and after electrocautery with Spetzler-Malis (e and f) Gross anatomical images before and after electrocautery with Silverglide. (Area of interest is marked in circle).
Tissue preparation and histological quantification of thermal injury
Transcardiac perfusion with physiological saline solution followed by 10% buffered formalin was performed after euthanasia following which the brain was harvested. Ex vivo fixation was performed in buffered formalin 10% for 2 weeks. The brain tissues were cut into three tissue blocks for each bipolar region tested, processed, and embedded in paraffin. Tissue sections of 5 µm thickness were obtained 20 µm apart using a microtome and stained with Hematoxylin and Eosin (H&E) per manufacturer instructions (Sigma-Aldrich, St. Louis, MO). Briefly, the tissues were deparaffinized and rehydrated using xylene and alcohol. The slides were then stained using H&E stain (Sigma-Aldrich, St. Louis, MO). The light microscope images were taken using Zeiss Axioskop 2 (Oberkochen, Germany) under ×5 magnification. The maximal depth of thermal injury was defined as the vertical line from cortical surface to the point of deepest injury and measured manually by Image J (National Institute of Health, Bethesda, Maryland). Similarly, area of injury was traced manually and quantified using with Image J (National Institute of Health, Bethesda, Maryland).[
Statistical analysis
Statistical analysis was performed using SPSS 22.0 (IBM Corporation, North Castle, NY) software. Maximal depth of injury and mean area of injury were compared across groups using one-way ANOVA followed by post hoc Tukey test for multiple group comparisons. All results were expressed as mean ± SEM. The criterion for statistical significance was set at P < 0.05.
RESULTS
Gross pathological examination
The craniectomy and cortical coagulation were completed without complications. Intraoperative images acquired during and after coagulation represent the extent of gross tissue damage and are displayed in [
Maximal depth of injury
The maximal depth of injury was significantly different among all groups (p<0.001). Multiple group comparison using post hoc Tukey test showed that TRIOwand cauterization resulted in significantly less depth of injury when compared to both Spetzler-Malis (TRIOwand: 421.8 ± 18.7 µm vs. SpetzlerMalis: 577.3 ± 21.7 µm; P < 0.001) and Silverglide cauterization (TRIOwand: 421.8 ± 18.7 µm vs. Silverglide: 483.5 ± 5.8 µm; P = 0.048). Silverglide cauterization caused significantly less injury depth compared to Spetzler-Malis cauterization (Silverglide: 483.5 ± 5.8 µm vs. Spetzler-Malis: 577.3 ± 21.7 µm; P < 0.001) and significantly greater depth of injury than the TRIOwand (Silverglide: 483.5 ± 5.8 µm vs. TRIOwand: 421.8 ± 18.7 µm; P = 0.048). The results are demonstrated in [
Figure 3:
Maximal depth of injury. (a) Spetzler-Malis bipolar forceps (b) NICO TRIOwand (c) Silverglide bipolar forceps. (d) TRIOwand cauterization resulted in significantly less depth of injury when compared to both Spetzler-Malis and Silverglide. Silverglide cauterization caused significantly less injury depth compared to Spetzler-Malis cauterization and significantly greater depth of injury than the TRIOwand.
Mean area of ınjury
The total area of injury differed significantly among all groups as well (p<0.001). Multiple group comparison using post hoc Tukey test showed that the injured area resulting from cauterization with the TRIOwand was significantly less than that of the Spetzler-Malis (TRIOwand: 489349.8 ± 31005.3 µm2 vs. Malis: 721129.6 ± 43269.9 µm2; P < 0.001) and the Silverglide (TRIOwand: 489349.8 ± 31005.3 µm2 vs. Silverglide: 719561.4 ± 10960.0 µm2; P < 0.001). Moreover, the areas of injury caused by the Spetzler-Malis and Silverglide systems were not significantly different than each other (Spetzler-Malis: 721129.6 ± 43269.9 µm2 vs. Silverglide: 719561.4 ± 10960.0 µm2; P = 0.99), the results of histological analysis are represented in [
Figure 4:
Mean area of injury. (a) Spetzler-Malis bipolar forceps (b) NICO TRIOwand (c) Silverglide bipolar forceps. (d) The injured area resulting from cauterization with the TRIOwand was significantly less than that of the Spetzler-Malis and the Silverglide. Moreover, the areas of injury caused by the Spetzler-Malis and Silverglide systems were not significantly different than each other.
Ischemic damage and rarefaction
All histological slides were assessed for evidence of rarefaction and ischemic injury. All three groups showed ischemic injury and rarefaction as demonstrated in [
DISCUSSION
The current study provides histological assessment of thermal injury in a porcine brain after bipolar electrocoagulation. The results demonstrate a greater depth and area of injury with Spetzler-Malis bipolar forceps as compared to Silverglide and TRIOwand forceps.
Several advances in bipolar cauterization technologies have been developed since its first introduction in 1940 by Greenwood. Significant improvements were made to bipolar technology by Dr. Leonard Malis with the introduction of the CMC I, II, and III systems.[
The use of different materials to minimize sticking and charring has also received attention. Hsiao et al. reported that two major issues in electro-surgery are thermal injury and tissue adherence and they found that the total area of injury of rat brain tissue treated with titanium dioxide layer-coated stainless steel type electrodes was significantly lower than that of untreated electrodes.[
Although much focus has been placed on bipolar electrode material and heat dissipation, an equally important principle of bipolar technology is the overall ergonomics. Current bipolars are designed keeping conventional open surgical approaches in mind. The bayoneted design is especially suited to neurosurgical dissection which involves working down deep, and narrow, surgical corridors while maintaining good visibility. This point is especially important as the advancement of robotic surgery into neurosurgery is demanding automation and multifunctional instrumentation from a single instrument, rather than the inefficiency of continuous instrument exchange. At our center, we have recently adopted port based technologies for parafascicular access to subcortical lesions.[
It should be noted that the absence of irrigation could certainly have impacted our results but we wanted to measure maximal damage and minimize differences across groups. It is also reasonable to hypothesize in a real world situation that the Spetzler-Malis bipolar forceps may simply be able to deal with troublesome bleeding faster and may be used for a shorter duration of time. Our study is not designed to answer this question and future studies will be needed to further address this issue. It is also unclear what these objective differences translate to from a clinical standpoint.
We selected swine as the animal model for this study given the size of its brain and spinal cord. Our goal was to assess thermal damage in anatomical structures that to an extent would mimic real life conditions in the operating room. In addition, pigs are an excellent large animal model in biomedical research since real-time measurement of physiological parameters including blood flow, temperature, tissue oxygenation, perfusion, and diffusion is possible. This can be quite difficult or sometimes impossible to monitor in genetically modified rodent models.
CONCLUSION
The TRIOwand forceps designed for port based access to deep subcortical and intraventricular pathology were not associated with significantly increased thermal injury when compared to the Spetzler-Malis and Silverglide systems in a swine model under nonhemorrhagic conditions using comparable and clinically relevant power settings. Further testing is required to validate these findings under hemorrhagic conditions in humans.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
This study was supported by NICO Corporation (NICO Corporation, Indianapolis, Indiana).
Conflicts of interest
There are no conflicts of interest.
References
1. Chen RK, Than KD, Wang AC, Park P, Shih AJ. Comparison of thermal coagulation profiles for bipolar forceps with different cooling mechanisms in a porcine model of spinal surgery. Surg Neurol Int. 2013. 4: 113
2. Elliott-Lewis EW, Benzel EC. Thermal comparison of novel bipolar forceps in bovine liver. Neurosurgery. 2010. 67: 160-4
3. Elliott-Lewis EW, Jolette J, Ramos J, Benzel EC. Thermal damage assessment of novel bipolar forceps in a sheep model of spinal surgery. Neurosurgery. 2010. 67: 166-71
4. Elliott-Lewis EW, Mason AM, Barrow DL. Evaluation of a new bipolar coagulation forceps in a thermal damage assessment. Neurosurgery. 2009. 65: 1182-7
5. Greenwood J. Two point coagulation: A follow-up report of a new technic and instrument for electrocoagulation in neurosurgery. Arch Phys Ther. 1942. 23: 552-4
6. Hsiao WT, Kung CM, Chu JS, Ou KL, Peng PW. Research of electrosurgical ablation with antiadhesive functionalization on thermal and histopathological effects of brain tissues in vivo. Biomed Res Int. 2014. 2014: 182657
7. Labib MA, Shah M, Kassam AB, Young R, Zucker L, Maioriello A. The safety and feasibility of image-guided brainpath-mediated transsulcul hematoma evacuation: A multicenter study. Neurosurgery. 2017. 80: 515-24
8. Malis LI. Electrosurgery and bipolar technology. Neurosurgery. 2006. 58: ONS1-12
9. Mikami T, Takahashi A, Hashi K, Gasa S, Houkin K. Performance of bipolar forceps during coagulation and its dependence on the tip material: A quantitative experimental assay. Technical note. J Neurosurg. 2004. 100: 133-8
10. Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012. 9: 671-5
11. Vellimana AK, Sciubba DM, Noggle JC, Jallo GI. Current technological advances of bipolar coagulation. Neurosurgery. 2009. 64: ons11-18