- Department of Neurosurgery, Shiga Medical Center for Adults, Shiga, Japan
- Shiga Medical Center Research Institute, Shiga, Japan
Correspondence Address:
Masato Hojo
Department of Neurosurgery, Shiga Medical Center for Adults, Shiga, Japan
DOI:10.4103/sni.sni_289_16
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Yoshito Sugita, Masahiro Sawada, Toshihiro Munemitsu, Tatsuya Higashi, Masato Hojo. Discrepancy between radiological and histological findings in ganglioglioma of the optic chasm: Case report. 11-Jul-2017;8:146
How to cite this URL: Yoshito Sugita, Masahiro Sawada, Toshihiro Munemitsu, Tatsuya Higashi, Masato Hojo. Discrepancy between radiological and histological findings in ganglioglioma of the optic chasm: Case report. 11-Jul-2017;8:146. Available from: http://surgicalneurologyint.com/surgicalint-articles/discrepancy-between-radiological-and-histological-findings-in-ganglioglioma-of-the-optic-chasm-case-report/
Abstract
Background:Gangliogliomas involving the optic nerve or chiasm are extremely rare tumors, which can be confused radiologically with other neoplasms. α-[N-methyl-11C]-methylaminoisobutyric acid (11C-MeAIB) is a new artificial amino acid positron emission tomography (PET) tracer, which is metabolically more stable in vivo and may be more specific for tumors than 11C-methionine. However, the utility of 11C-MeAIB PET in the diagnosis of brain tumors has not yet been reported.
Case Description:A 26-year-old man presented with visual field defects and headache, and magnetic resonance imaging demonstrated a suprasellar mass involving the optic chiasm. A biopsy and partial tumor resection were performed via an interhemispheric approach. We diagnosed the tumor as ganglioglioma (WHO grade I) involving the optic chiasm. Although this lesion was histologically benign, 11C-MeAIB PET, 2-deoxy-2-[18F] fluoro-D-glucose (18F-FDG) PET and proton magnetic resonance spectroscopy indicated malignant features.
Conclusion:The discrepancy between radiological and histological findings implies that this new amino acid tracer PET may have a limitation in the diagnosis of gangliogliomas. Although further study is necessary, gangliogliomas should be included in the differential diagnosis of suprasellar tumors, even if PET findings show malignant features.
Keywords: Ganglioglioma, MeAIB PET, optic chiasm
INTRODUCTION
Gangliogliomas, which are composed of both neuronal and glial components, are rare and account for only 0.3–1.3% of all brain tumors.[
α-[N-methyl-11C]-methylaminoisobutyric acid (11C-MeAIB) is a new artificial amino acid PET tracer, which is metabolically more stable in vivo and may be more specific for tumors than 11C-methionine (11C-MET). However, the utility of 11C-MeAIB PET in the diagnosis of brain tumors has not yet been reported. Here, we report the first case of ganglioglioma of the optic chiasm, which was evaluated by 11C-MeAIB PET, 2-deoxy-2-[18F] fluoro-D-glucose (18F-FDG) PET and MRS. We also show discrepancies between radiological and histological findings in this lesion.
CASE DESCRIPTION
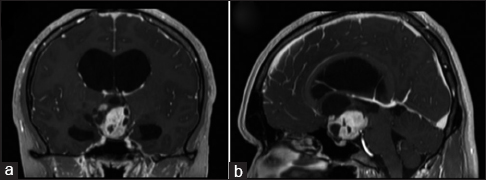
A 26-year-old man presented with a 1-month history of progressive visual field defects and headache. Neuro-ophthalmological examination revealed temporal hemianopsia and bilateral papilledema. As part of the diagnostic work-up at our institution, computed tomography (CT), magnetic resonance imaging (MRI), MRS (Ingenia 3.0T, Philips Medical System, Amsterdam, Nederland), and PET (GE Advance, GE Healthcare, Waukesha WI, USA) were performed. CT revealed a mixed-dense suprasellar mass lesion without signs of calcification, as well as hydrocephalus caused by obstruction of the foramina of Monro. MRI demonstrated a suprasellar mass involving the optic chiasm, which had cystic components. The lesion was hypointense on T1-weighted images (WI), hyperintense on T2-WI, and showed marked enhancement after the administration of contrast material [
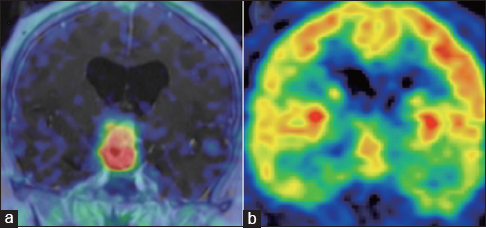
11C-MeAIB PET revealed remarkably high uptake of MeAIB] standardized uptake value (SUV) max = 9.8] consistent with enhanced lesion on MRI, and lesion to gray matter ratio (LGR) was 56.9 [
A biopsy and partial tumor resection were performed via an interhemispheric approach. The intraoperative findings were that the tumor originated from the optic chiasm without a clear plane of dissection. Therefore, total resection was abandoned to avoid damage to the optic nerve. Histopathological evaluation of the tumor revealed that the tumor comprised both neoplastic glial and ganglion cells. Immunohistochemical staining demonstrated that dysmorphic ganglion cells were positive for synaptophysin, and pleomorphic glial cells were positive for glial fibrillary acid protein (GFAP). No high-grade neoplastic feature was detected, and Ki-67 immunoreactivity was less than 3%. We diagnosed the tumor as ganglioglioma (WHO grade I) involving the optic chiasm. Although 11C-MeAIB PET, 18F-FDG PET, and MRS indicated malignant features, the lesion was histologically benign. Because ventricular dilatation did not improve, a ventriculoperitoneal shunt was performed. Postoperative course was uneventful. Although the role of postoperative radiotherapy is controversial, most cases of optic gangliogliomas reported in the literature have received adjuvant radiation when only biopsy or subtotal resection could be performed.[
DISCUSSION
Gangliogliomas of the optic chiasm are extremely rare and radiologically difficult to distinguish from other suprasellar tumors.[
It is well known that 11C-MET PET is more sensitive for brain tumors than 18F-FDG-PET. Im et al. have reported a case of cerebral ganglioglioma, which showed hypometabolism on 11C-MET PET.[
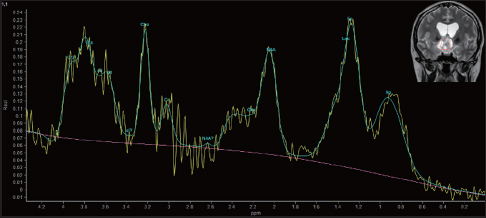
MRS provides useful information on tumor metabolism and has been proposed as a method for tumor grading.[
CONCLUSIONS
We have shown the first case of ganglioglioma of the optic chiasm, which was evaluated by 11C-MeAIB PET, 18F-FDG PET, and MRS. Although this lesion was histologically benign, 11C-MeAIB PET, 18F-FDG PET, and MRS indicated malignant features. Since this report shows only one case, further study is necessary to confirm the advantages and limitations of these diagnostic modalities. Gangliogliomas should be included in the differential diagnosis of suprasellar tumors, even if PET and MRS findings show malignant features.
Disclosure
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
We thank Shigeru Tanaka, Miki Ito, Yusuke Chaya, and Takuma Hayashi for technical help.
References
1. Fink JR, Carr RB, Matsusue E, Iyer RS, Rockhill JK, Haynor DR. Comparison of 3 Tesla proton MR spectroscopy, MR perfusion and MR diffusion for distinguishing glioma recurrence from posttreatment effects. J Magn Reson Imaging. 2012. 35: 56-63
2. Hwang JH, Egnaczyk GF, Ballard E, Dunn RS, Holland SK, Ball WS. Proton MR spectroscopic characteristics of pediatric pilocytic astrocytomas. AJNR Am J Neuroradiol. 1998. 19: 535-40
3. Im SH, Chung CK, Cho BK, Wang KC, Yu IK, Song IC. Intracranial ganglioglioma: Preoperative characteristics and oncologic outcome after surgery. J Neuro-oncol. 2002. 59: 173-83
4. Kincaid PK, El-Saden SM, Park SH, Goy BW. Cerebral gangliogliomas: Preoperative grading using FDG-PET and 201Tl-SPECT. AJNR Am J Neuroradiol. 1998. 19: 801-6
5. Kumabe T, Shimizu H, Sonoda Y, Shirane R. Thallium-201 single-photon emission computed tomographic and proton magnetic resonance spectroscopic characteristics of intracranial ganglioglioma: Three technical case reports. Neurosurgery. 1999. 45: 183-7
6. Liu GT, Galetta SL, Rorke LB, Bilaniuk LT, Vojta DD, Molloy PT. Gangliogliomas involving the optic chiasm. Neurology. 1996. 46: 1669-73
7. Löbel U, Ellison DW, Shulkin BL, Patay Z. Infiltrative cerebellar ganglioglioma: Conventional and advanced MRI, proton MR spectroscopic, and FDG PET findings in an 18-month-old child. Clinical Radiol. 2011. 66: 194-201
8. Meyer PT, Spetzger U, Mueller HD, Zeggel T, Sabri O, Schreckenberger M. High F-18 FDG uptake in a low-grade supratentorial ganglioma: A positron emission tomography case report. Clin Nucl Med. 2000. 25: 694-7
9. Nishii R, Higashi T, Kagawa S, Kishibe Y, Takahashi M, Yamauchi H. Diagnostic usefulness of an amino acid tracer, α-[N-methyl-11C]-methylaminoisobutyric acid (11C-MeAIB), in the PET diagnosis of chest malignancies. Ann Nucl Med. 2013. 27: 808-21
10. Phi JH, Paeng JC, Lee HS, Wang KC, Cho BK, Lee JY. Evaluation of focal cortical dysplasia and mixed neuronal and glial tumors in pediatric epilepsy patients using 18F-FDG and 11C-methionine pet. J Nucl Med. 2010. 51: 728-34
11. Rolston JD, Han SJ, Cotter JA, El-Sayed IH, Aghi MK. Gangliogliomas of the optic pathway. J Clin Neurosci. 2014. 21: 2244-9
12. Saga T, Kawashima H, Araki N, Takahashi JA, Nakashima Y, Higashi T. Evaluation of primary brain tumors with FLT-PET: Usefulness and limitations. Clin Nucl Med. 2006. 31: 774-80
13. Shuangshoti S, Kirsch E, Bannan P, Fabian VA. Ganglioglioma of the optic chiasm: Case report and review of the literature. AJNR Am J Neuroradiol. 2000. 21: 1486-9
14. Spennato P, Giordano M, Ruggiero C, Aliberti F, Buonocore MC, Nastro A. Optic pathway ganglioglioma with intraventricular cyst. J Neuro-oncol. 2011. 102: 499-508
15. Sutinen E, Jyrkkio S, Alanen K, Nagren K, Minn H. Uptake of [N-methyl-11C] alpha-methylaminoisobutyric acid in untreated head and neck cancer studied by PET. Eur J Nucl Med Mol Imaging. 2003. 30: 72-7