- Health Science Center, University of Fortaleza, Ceara, Brazil.
- Department of Neurosurgery, General Hospital of Fortaleza, Fortaleza, Ceara, Brazil.
Correspondence Address:
Leonardo Jose Monteiro de Macedo Filho
Department of Neurosurgery, General Hospital of Fortaleza, Fortaleza, Ceara, Brazil.
DOI:10.25259/SNI_586_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Leonardo Jose Monteiro de Macedo Filho1, Esther Grangeiro Barreto1, Paulo Levi Bezerra Martins1, Euler Nicolau Sauaia Filho1, Gunter Gerson2, Lucas Alverne Freitas de Albuquerque2. IDH1-mutant primary intraventricular gliosarcoma: Case report and systematic review of a rare location and molecular profile. 06-Nov-2020;11:372
How to cite this URL: Leonardo Jose Monteiro de Macedo Filho1, Esther Grangeiro Barreto1, Paulo Levi Bezerra Martins1, Euler Nicolau Sauaia Filho1, Gunter Gerson2, Lucas Alverne Freitas de Albuquerque2. IDH1-mutant primary intraventricular gliosarcoma: Case report and systematic review of a rare location and molecular profile. 06-Nov-2020;11:372. Available from: https://surgicalneurologyint.com/surgicalint-articles/10377/
Abstract
Background: Gliosarcoma (GS) is classified as an IDH-wild-type variant of glioblastoma (GBM). While GS is already an unusual presentation of GBM, IDH1-mutant cases are especially rare. We present an IDH1-mutant primary intraventricular GS case report and a systematic review of the molecular profile in GS correlating to the prognostic and pathogenesis of IDH1/2 mutations.
Case Description: A 44-years-old man presented with ongoing fatigue symptoms and a new-onset intense occipital headache. The patient complained of memory loss, dyscalculia, and concentration difficulties. An MRI revealed a bihemispheric intraventricular mass crossing the midline through the corpus callosum and infiltrating the trigone of the lateral ventricles, hypointense, and hyperintense on the T1- and T2-weighted image. We performed a microsurgical resection with a transparietal transsulcal approach; however, the contralateral mass was attached to vascular structures and we decided to reoperate the patient in another moment. The histopathological study showed a Grade IV tumor and the immunohistochemistry confirmed the diagnosis of GS. The patient presented progressive neurologic decline and died 45 days after the surgical approach.
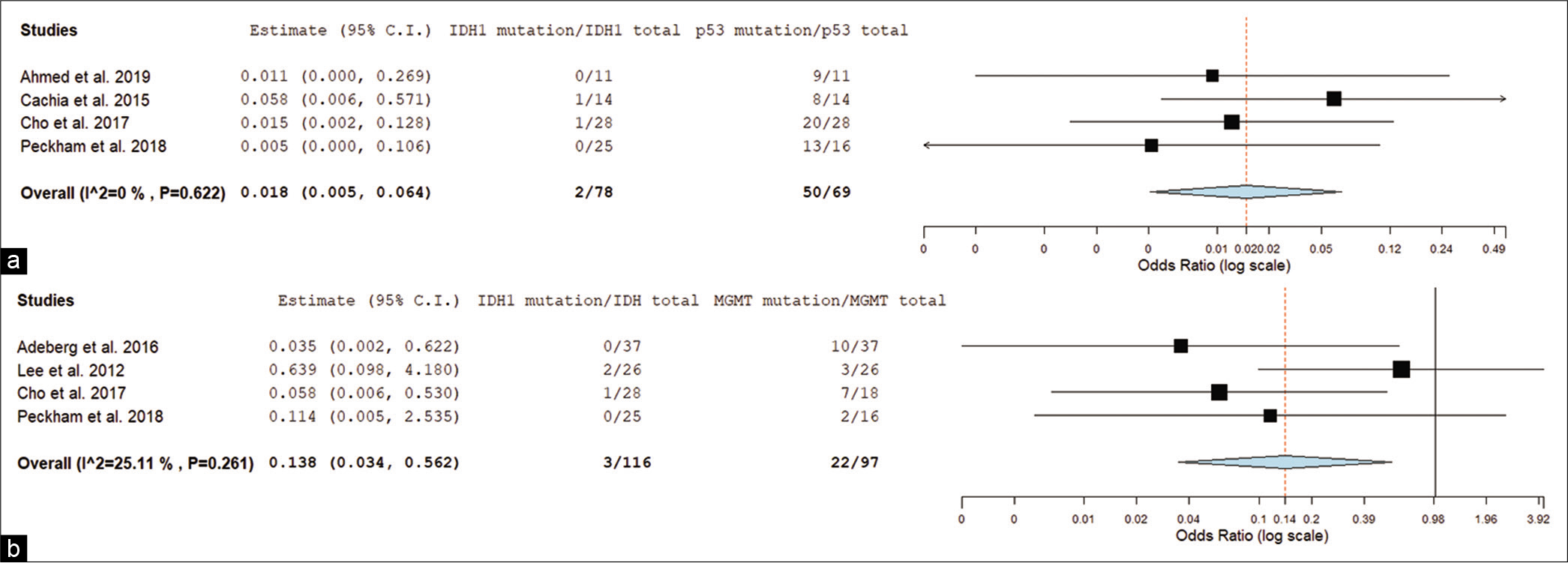
Conclusion: We did two systematic reviews studies from PubMed, EMBASE, MEDLINE, Cochrane, and SCOPUS databases, and included molecular and intraventricular studies of GS. We performed further meta-analysis using OpenMetaAnalyst™ software. We conducted a forest plot with the molecular profile of GS. When correlated IDH1 mutation versus tp53 mutation, we found an odds ratio (OR) of 0.018 (0.005–0.064) and P P = 0.006; OR = 0.138 [0.034–0.562]). The studies evaluating the molecular profile in GS prognostics are often extended from all GBMs despite specifics GBM variants (i.e., GS). We found a correlation between IDH1 mutation expression with tp53 and MGMT expression in GS, and future studies exploring this molecular profile in GS are strongly encouraged.
Keywords: Case report, Cerebral ventricle neoplasms, Gliosarcoma, Human IDH1 protein, Systematic review
INTRODUCTION
Gliosarcomas (GS) are rare primary high-grade brain tumors and a variant of GBM, constituting 2-8% of all GBM.[
A modest propensity for temporal lobe involvement was observed in GS followed by the frontal, parietal, and occipital lobes. Cerebellum, pineal region, cerebellopontine angle, intraventricular, and spinal cord have been described as rare primary locations for these lesions.[
Headache was the most common presentation of intraventricular GS, other clinical symptoms including aphasia hemiparesis, seizures, and cognitive decline depends on location, size of the tumor, and the existence of hydrocephalus.[
We present a rare case of an IDH1-mutant primary intraventricular GS and a systematic review of the molecular profile in GS correlating to the prognostic and pathogenesis of IDH1/2 mutations.
CASE REPORT
Patient
A 44-years-old man presented with ongoing fatigue symptoms and a new-onset intense occipital headache which was worse in the night and was waking him up from sleep. The patient reported progressive deterioration of the symptoms with increased intensity and frequency of the headaches; moreover, he complained of memory loss, dyscalculia, concentration difficulties, psychomotor agitation, and aggressive behavior.
He was initially treated with dipyrone (4 g/day, oral), naproxen sodium (550 mg/day, oral), and dexamethasone (24 mg/day, oral) from urgent care, and referred to the neurosurgery department. He presented a normal mental status (GCS 15) without impairment on his physical examination and mild bilateral papilledema on his neurological examination.
Imaging
It was performed a brain MRI [
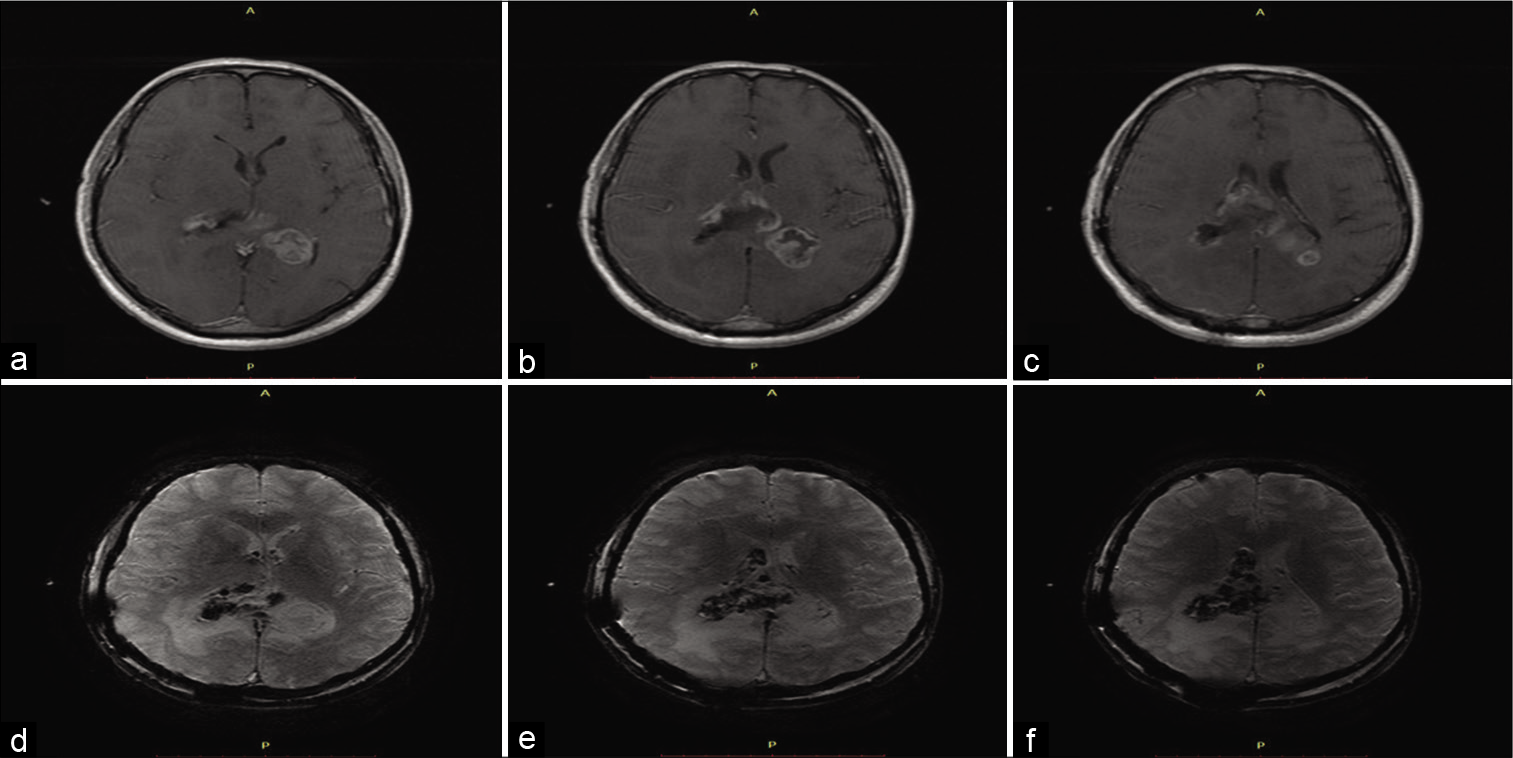
Figure 1:
Preoperative MRI dated 1 month before surgery. (a) Axial T1WI MRI showing extensive amorphic heterogeneous mass invading both lateral ventricles with a commitment of midline. (b) Axial T1WI Gd MRI demonstrates the same lesion with ring and internal enhancement. (c) Coronal T1WI Gd MRI showing better the internal enhancement and commitment of both lateral ventricles. (d) Axial T2WI MRI exhibiting heterogeneous intratumoral signal and irregular-margin enhancement. Note hypointense signal surrounding the lesion suggesting extensive vasogenic edema. (e) Axial DWI shows nonimpaired diffusion. (f) ADC Map demonstrating high signal.
The volume of the lesion (manual segmentation) was 63.1 cc and the estimated mean diameter was 50.15 mm. The initial hypothesis was GBM or anaplastic ependymoma. We discussed with the patient about the option of stereotactic biopsy to obtain samples for diagnostic purposes. However, the patient opted for microsurgery for maximum resection of the lesion; however, the gross-total resection was not achievable due to tumor extension.
Surgery
We decided to perform a microsurgical resection with a right transparietal transsulcal approach, reaching the trigone of the right ventricle and the infiltrative mass. We resected the ipsilateral brain lesion; however, the contralateral ventricle resection was limited due to a deep surgical corridor and the need to manipulate vascular structures (i.e., vein of Galen). The immediate postoperative MRI revealed a residual tumor volume of 14.03 cc (estimated mean diameter of 30.58 mm) in the left ventricular trigone [
Figure 2:
Immediate postoperative Control MRI. (a-c) T1WI Gd MRI exhibiting residual mass on the left ventricle atrium. (d-f) T2WI MRI demonstrating residual mass on the left ventricle atrium. The inclusion criteria in our first systematic review were case series studies with at least ten patients containing GS with molecular profile study (IDH1/2, ATRX, tp53, TERT, 1p19q, or Ki-67). Cases series without any molecular profile were excluded from the study.
Figure 3:
(a) Sarcomatous component, with marked pleomorphic spindle cells and mitotic activity (H and E, ×10). (b) Glial component, presenting hypercellularity, pleomorphism, mitotic figures, and nuclear atypia (H and E, ×20). (c) Glial component. Featuring hypercellularity, a high degree of anaplasia, presence of bizarre multinucleated cells, nuclear atypia, and evident mitotic figures (H and E, ×40). (d) Sarcomatous component, presenting mitotic figures, and nuclear atypia (H and E, ×20).
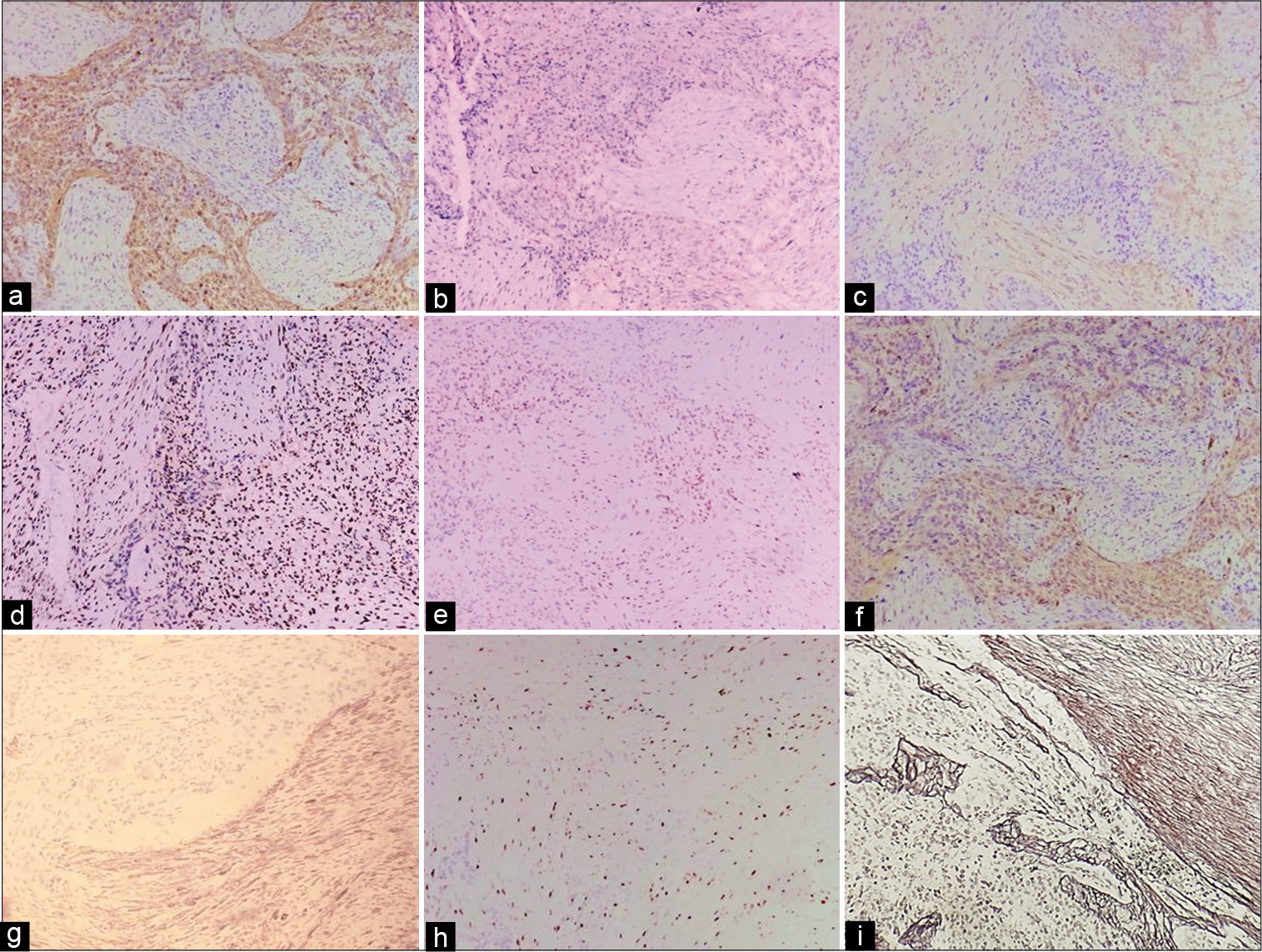
Figure 4:
Immunohistochemical stains. (a) Focal positivity for GFAP, only in glial component (×10). (b) IDH was positive in the glial component (×10). (c) SMA (Smooth Muscle Actin) was positive in the sarcomatous component (×10). (d) Partial loss of ATRX expression (intact) in tumor cells (×10). (e) S100 was positive in the glial component (×10). (f) Vimentin was positive in the sarcomatous component (×20). (g) Diffuse positivity for p53 stain - approximately 80% of neoplastic cells (×10). (h) Ki-67 stain showed more than 60% proliferative activity in the tumor nuclei - 35% of neoplastic cells (×10). (i) Gomori silver stain highlights reticulin, negative in the glial component, and positive in the sarcomatous component.
Postoperative evaluation
After 20 days of the procedure, the patient presented an improvement of headache and psychomotor agitation; however, he continued with progressive worsening of memory loss and showed a diminished spatial awareness.
We started adjuvant radiotherapy and chemotherapy with temozolomide. However, one month after the tumor resection, a new MRI revealed important residual lesion growing on the trigone of the left ventricle with a tumor volume of 41.6 cc (estimated mean diameter of 43.65 mm) and an impressive growth rate estimated in 176.68 mm/year [
MATERIALS AND METHODS
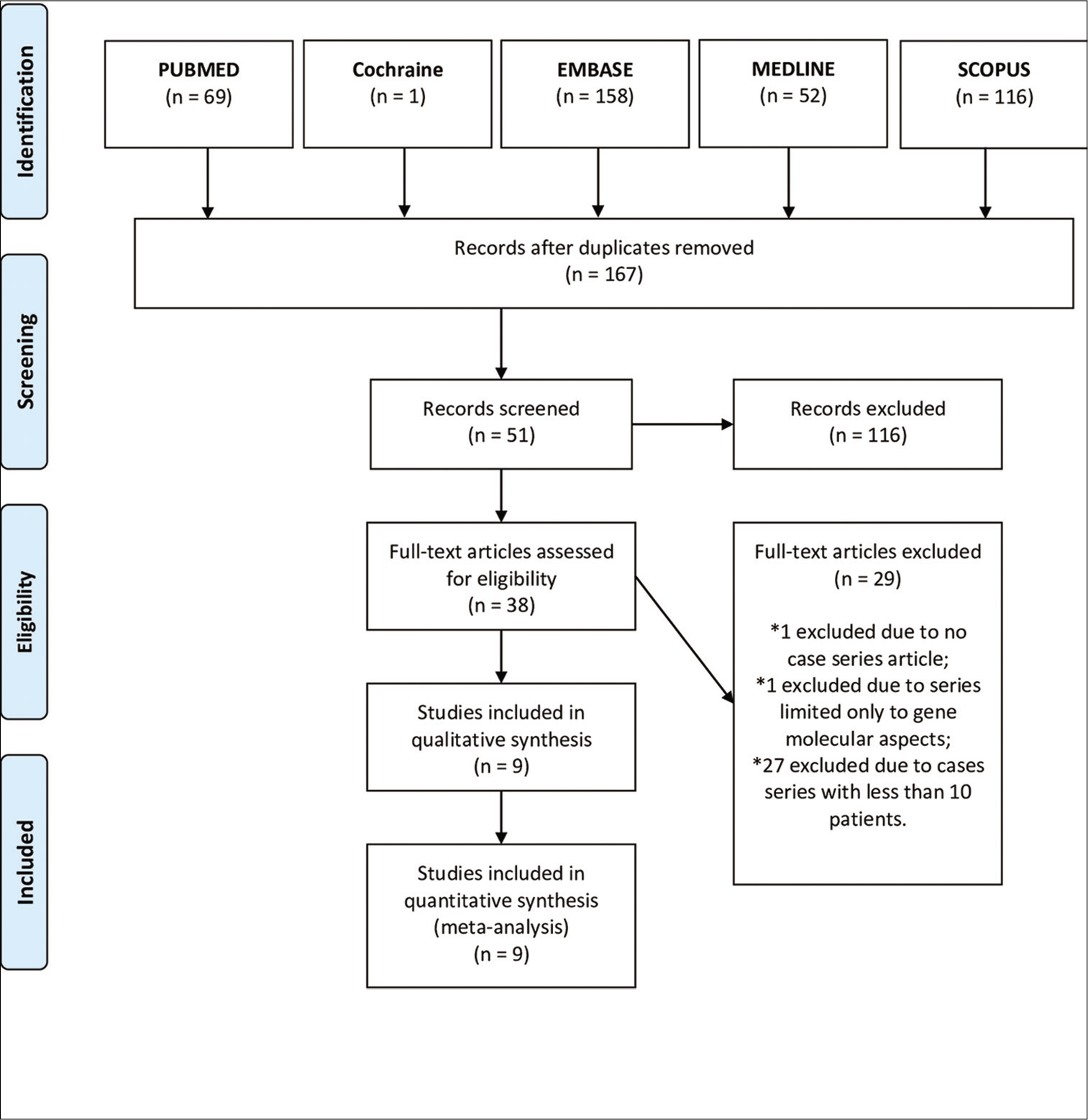
We performed two systematic reviews of the literature using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and protocol. A literature search was performed using PubMed, EMBASE, Ovid MEDLINE, Cochrane Library, and SCOPUS databases. Search terms included (GS) AND [(idh1) OR (idh2) OR (atrx) OR (p53) OR (tert) OR (1p19q) OR (Ki-67)] in our first systematic review [
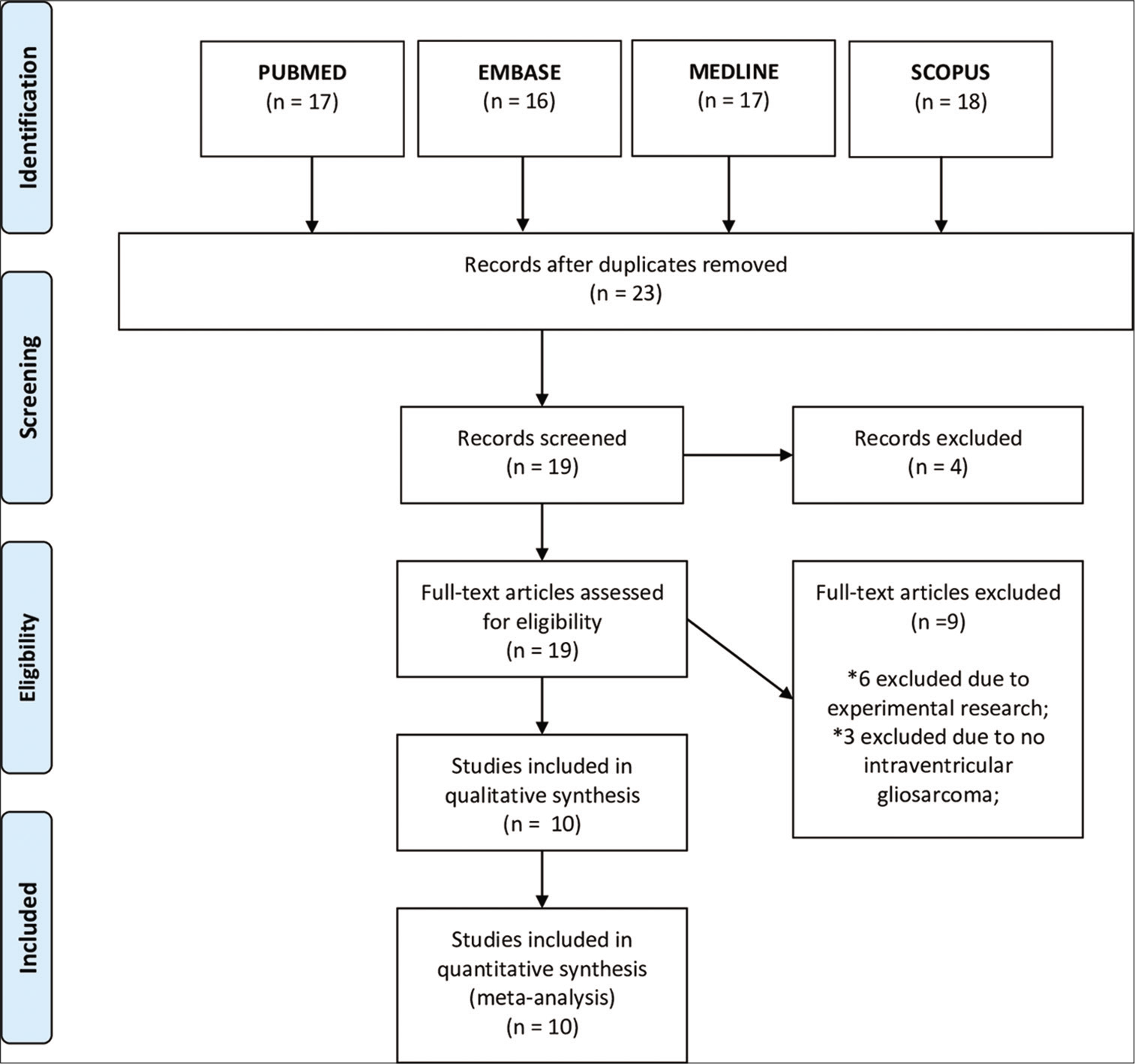
In our second review, we included only case series studies containing primary GS in the intraventricular location, the exclusion criteria were case series without exclusive GS intraventricular location.
Included studies were assessed by two authors (L. J. M. M. F and L. A. F. A.) to ensure that cases were correctly included in the study. Patient data from multiple studies were combined into two tables for comparison [
We used the maximal tumor diameter as a parameter of a possible outcome. In this case, we transformed the tumor volume (V) in an equivalent mean tumor diameter (MTD) using the formula [MTD = (2 × V)1/3] to standardize our study.[
The OpenMetaAnalyst™ meta-analysis software (Brown University, RI, USA) was used to perform a forest plot correlating IDH1 versus tp53 and IDH1 versus MGMT methylations in the case series of [
RESULTS
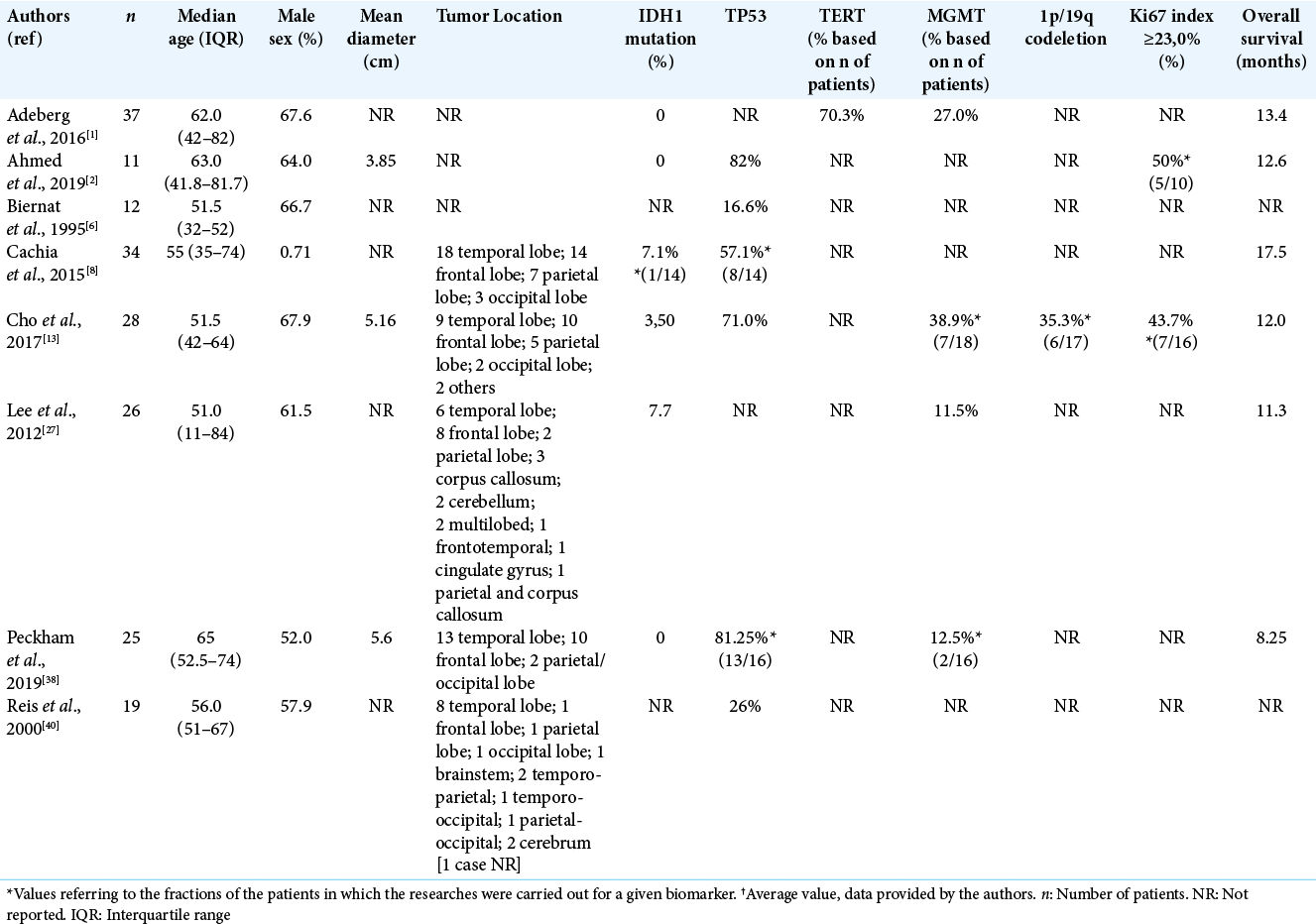
A total of 8 series were included in our first systematic review focused mainly on GS molecular signature. A total of 192 patients were identified [
Regarding the GS molecular profile studies found in the collected articles, we identified IDH1 mutations in 5.88% (n = 4/68) and TP53 mutations in 57% (n = 57/100) of patients. TERT mutations and 1p/19q codeletion were reported, respectively, in 70.3% (n = 26/37) and 35.3% (n = 6/17) of patients. Ki-67 index ≥23% was measured in 46.15% (n = 12/26) of evaluated patients. Methylated MGMT was identified in 22.68% (n = 22/97) of the patients. The mean OS of these patients was 12.51 (± 3.02) months and the median was 12.3 months.
We conducted a forest plot with the molecular profile of GS [
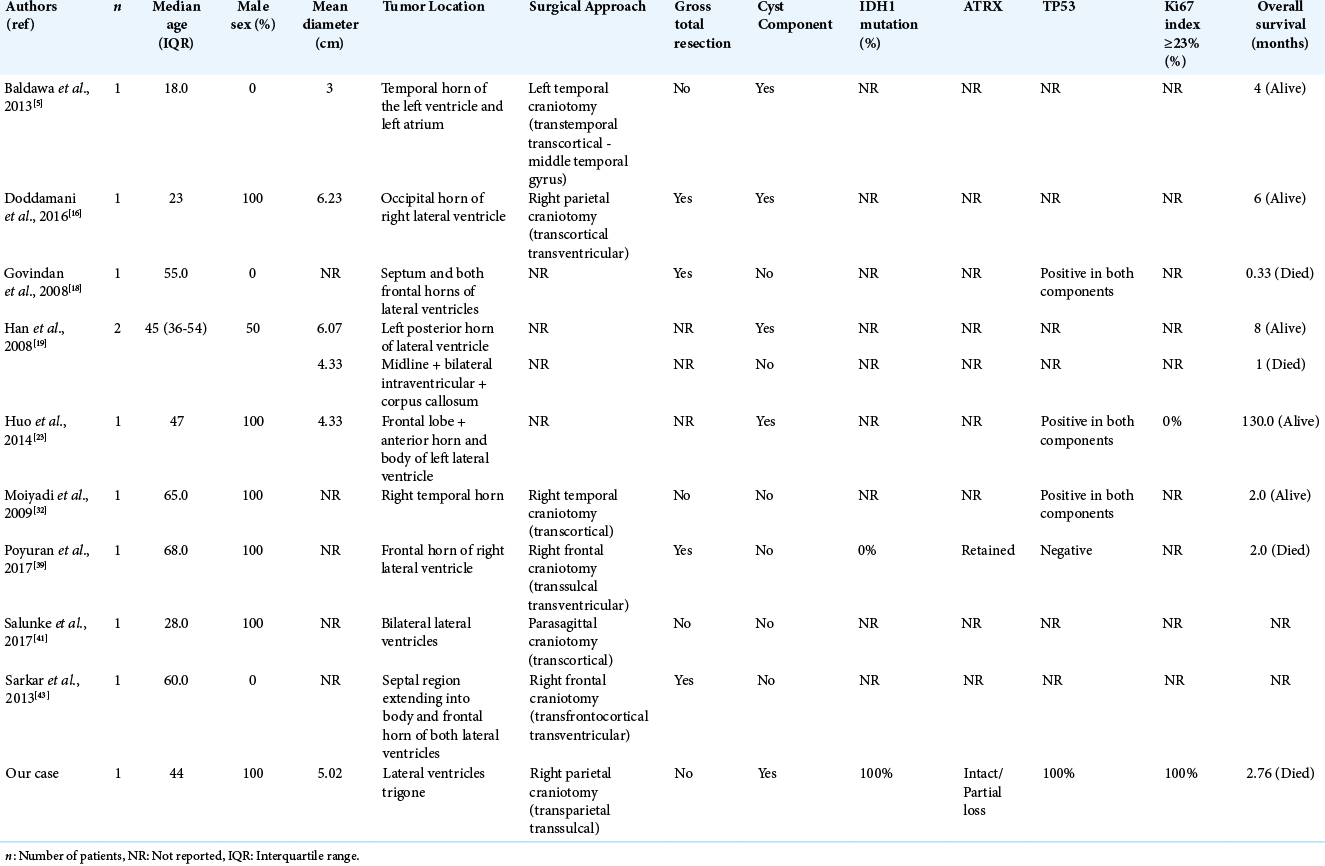
Our second systematic review included a total of ten intraventricular GS and we included our case for further evaluation [
DISCUSSION
IDH1/2 has an important role in chemo and/or radiotherapy in many types of tumors,[
Both components (i.e., gliomatous and sarcomatous) of GS present tp53 mutations or overexpression and are found in patients with primary and secondary GS, suggesting that they may occur early in gliomagenesis.[
Some studies suggest that ATRX is expressed in all GS.[
TERT promoter mutations were majorly present in both glial and mesenchymal tumor areas in GS, and they play a crucial role by conferring these tumors unrestricted growth properties, contributing to the tumorigenesis.[
Ki-67 is a nonhistone nuclear protein and a cellular marker associated with ribosomal RNA transcription in cell proliferation[
MGMT is a DNA repair protein and its loss is correlated to increased survival in malignant gliomas.[
In our case, the tumor was in the atrium and the occipital horn of the lateral ventricles and we decided to achieve a maximal resection minimizing the risks to the relevant subcortical tracts through a transsulcal approach. A gross total resection of the tumor without significant complication requires a thorough understanding of available surgical approaches and their relative advantages and disadvantages.[
Limitations
We may point some relevant limitations in our paper.
Since there are many nonrecorded IDH1 statuses in prior studies in both overall and intraventricular group, the percentages as described may have errors. Although we found a statistical correlation between IDH1 mutation and tp53 and between IDH1 mutation and MGMT, we must alert that with so few numbers of IDH1 positive cases data might be erroneous.
Due to the great limitation of data related to GS in the literature, many comparisons and analogies in the discussion were made in relation to GBM, including the cutoff point used for the Ki-67 of 23%, which is not entirely adequate because they are distinct pathologies, despite some similarities.
CONCLUSION
There is a lack of data in the literature related to molecular profiles specific to GS, with an inappropriate tendency to compare their behavior and molecular profile with GBM. More molecular studies are needed for GS.
We found a correlation between IDH1 mutation expression with p53 and MGMT expression in GS, and future studies exploring this molecular profile in GS are strongly encouraged.
Our study validates the need to perform IDH1 analysis in all GS cases and assess other molecular and clinical associations and outcomes, respectively.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Adeberg S, Bernhardt D, Harrabi SB, Diehl C, Koelsche C, Rieken S. Radiotherapy plus concomitant temozolomide in primary gliosarcoma. J Neurooncol. 2016. 128: 341-8
2. Ahmed FI, Abdullah KG, Durgin J, Salinas RD, O’Rourke DM, Brem S. Evaluating the association between the extent of resection and survival in gliosarcoma. Cureus. 2019. 11: e4374
3. Alkhaibary A, Alassiri AH, AlSufiani F, Alharbi MA. Ki-67 labeling index in glioblastoma; does it really matter?. Hematol Oncol Stem Cell Ther. 2019. 12: 82-8
4. Arita H, Narita Y, Fukushima S, Tateishi K, Matsushita Y, Yoshida A, Miyakita Y. Upregulating mutations in the TERT promoter commonly occur in adult malignant gliomas and are strongly associated with total 1p19q loss. Acta Neuropathol. 2013. 126: 267-76
5. Baldawa S, Kasegaonkar P, Vani S, Kelkar G. Primary intraventricular gliosarcoma. Clin Neuropathol. 2013. 32: 525-8
6. Biernat W, Aguzzi A, Sure U, Grant JW, Kleihues P, Hegi ME. Identical mutations of the p53 tumor suppressor gene in the gliomatous and the sarcomatous components of gliosarcomas suggest a common origin from glial cells. J Neuropathol Exp Neurol. 1995. 54: 651-6
7. Boots-Sprenger SH, Sijben A, Rijntjes J, Tops BB, Idema AJ, Rivera AL. Significance of complete 1p/19q co-deletion, IDH1 mutation and MGMT promoter methylation in gliomas: Use with caution. Mod Pathol. 2013. 26: 922-9
8. Cachia D, Kamiya-Matsuoka C, Mandel JJ, Olar A, Cykowski MD, Armstrong TS. Primary and secondary gliosarcomas: Clinical, molecular and survival characteristics. J Neurooncol. 2015. 125: 401-10
9. Cai J, Chen J, Zhang W, Yang P, Zhang C, Li M. Loss of ATRX, associated with DNA methylation pattern of chromosome end, impacted biological behaviors of astrocytic tumors. Oncotarget. 2015. 6: 18105-15
10. Cai J, Yang P, Zhang C, Zhang W, Liu Y, Bao Z. ATRX mRNA expression combined with IDH1/2 mutational status and Ki-67 expression refines the molecular classification of astrocytic tumors: Evidence from the whole transcriptome sequencing of 169 samples samples. Oncotarget. 2014. 5: 2551-61
11. Cambruzzi E. The role of IDH1/2 mutations in the pathogenesis of secondary glioblastomas. J Bras de Patol Med Lab. 2017. 53: 338-44
12. Chaurasia A, Park SH, Seo JW, Park CK. Immunohistochemical analysis of ATRX, IDH1 and p53 in glioblastoma and their correlations with patient survival. J Korean Med Sci. 2016. 31: 1208-14
13. Cho SY, Park C, Na D, Han JY, Lee J, Park OK. High prevalence of TP53 mutations is associated with poor survival and an EMT signature in gliosarcoma patients. Exp Mol Med. 2017. 49: e317
14. Cikla U, Swanson KI, Tumturk A, Keser N, Uluc K, Cohen-Gadol A. Microsurgical resection of tumors of the lateral and third ventricles: Operative corridors for difficult-to-reach lesions. J Neurooncol. 2016. 130: 331-40
15. Souza LC, Nicoletti AL, Leão CM, de Lima IT, de Souza JT, Coutinho SL. Long-term metastatic gliosarcoma survival after meningioma resection: Case report and literature revision. J Bras Neurocir. 2017. 28: 92-100
16. Doddamani RS, Meena RK, Selvam MM, Venkataramanaa NK, Tophkhane M, Garg SK. Intraventricular gliosarcomas: Literature review and a case description. World Neurosurg. 2016. 90: 707.e5-12
17. Frandsen J, Orton A, Jensen R, Colman H, Cohen AL, Tward J. Patterns of care and outcomes in gliosarcoma: An analysis of the national cancer database. J Neurosurg. 2018. 128: 1133-8
18. Govindan A, Bhat DI, Mahadevan A, Chakraborti S, Sampath S, Chandramouli BA. An unusual case of intraventricular gliosarcoma. Clin Neuropathol. 2009. 28: 379-83
19. Han L, Zhang X, Qiu S, Li X, Xiong W, Zhang Y. Magnetic resonance imaging of primary cerebral gliosarcoma: A report of 15 cases. Acta Radiol. 2008. 49: 1058-67
20. Han SJ, Yang I, Tihan T, Chang SM, Parsa AT. Secondary gliosarcoma: A review of clinical features and pathological diagnosis. J Neurosurg. 2010. 112: 26-32
21. Han SJ, Yang I, Tihan T, Prados MD, Parsa AT. Primary gliosarcoma: Key clinical and pathologic distinctions from glioblastoma with implications as a unique oncologic entity. J Neurooncol. 2010. 96: 313-20
22. Huang Q, Li F, Chen Y, Hong F, Wang H, Chen J. Prognostic factors and clinical outcomes in adult primary gliosarcoma patients: A surveillance, epidemiology, and end results (SEER) analysis from 2004 to 2015. Br J Neurosurg. 2020. 34: 161-7
23. Huo Z, Yang D, Shen J, Li Y, Wu H, Meng Y. Primary gliosarcoma with long-survival: Report of two cases and review of literature. Int J Clin Exp Pathol. 2014. 7: 6323-32
24. Kakkar N, Kaur J, Singh GK, Singh P, Siraj F, Gupta A. Gliosarcoma in young adults: A rare variant of glioblastoma. World J Oncol. 2017. 8: 53-7
25. Kang SH, Park KJ, Kim CY, Yu MO, Park CK, Park SH. O6-methylguanine DNA methyltransferase status determined by promoter methylation and immunohistochemistry in gliosarcoma and their clinical implications. J Neurooncol. 2011. 101: 477-86
26. Kozak KR, Mahadevan A, Moody JS. Adult gliosarcoma: Epidemiology, natural history, and factors associated with outcome. Neuro Oncol. 2009. 11: 183-91
27. Lee D, Kang SY, Suh YL, Jeong JY, Lee JI, Nam DH. Clinicopathologic and genomic features of gliosarcomas. J Neurooncol. 2012. 107: 643-50
28. Lee Y, Koh J, Kim SI, Won JK, Park CK, Choi SH. The frequency and prognostic effect of TERT promoter mutation in diffuse gliomas. Acta Neuropathol Commun. 2017. 5: 62
29. Louis DN, Perry A, Reifenberger G, von Deimling A, FigarellaBranger D, Cavenee WK. The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016. 131: 803-20
30. Lowder L, Hauenstein J, Woods A, Chen HR, Rupji M, Kowalski J. Gliosarcoma: Distinct molecular pathways and genomic alterations identified by DNA copy number/SNP microarray analysis. J Neurooncol. 2019. 143: 381-92
31. Ludwig K, Kornblum HI. Molecular markers in glioma. J Neurooncol. 2017. 134: 505-12
32. Moiyadi A, Sridhar E, Jalali R. Intraventricular gliosarcoma: Unusual location of an uncommon tumor. J Neurooncol. 2010. 96: 291-4
33. Molenaar RJ, Maciejewski JP, Wilmink JW, van Noorden CJ. Wild-type and mutated IDH1/2 enzymes and therapy responses. Oncogene. 2018. 37: 1949-60
34. Ning L, Wang PF, Song HW, Kong LW, Yao K, Qi XL. Immunostaining of IDH-1 R132H and ATRX proteins in the classification of adult glioblastomas. Int J Clin Exp Pathol. 2016. 9: 12849-54
35. Oh JE, Ohta T, Nonoguchi N, Satomi K, Capper D, Pierscianek D. Genetic alterations in gliosarcoma and giant cell glioblastoma. Brain Pathol. 2016. 26: 517-22
36. Pain M, Wang H, Lee E, Strahl M, Hamou W, Sebra R. Treatment-associated TP53 DNA-binding domain missense mutations in the pathogenesis of secondary gliosarcoma. Oncotarget. 2017. 9: 2603-21
37. Pallud J, Taillandier L, Capelle L, Fontaine D, Peyre M, Ducray F. Quantitative morphological magnetic resonance imaging follow-up of low-grade glioma: A plea for systematic measurement of growth rates. Neurosurgery. 2012. 71: 729-40
38. Peckham ME, Osborn AG, Palmer CA, Tsai A, Salzman KL. Gliosarcoma: Neuroimaging and immunohistochemical findings. J Neuroimaging. 2019. 29: 126-32
39. Poyuran R, Bn N, Reddy YV, Savardekar AR. Intraventricular gliosarcoma with dual sarcomatous differentiation: A unique case. Neuropathology. 2017. 37: 346-50
40. Reis RM, Könü-Lebleblicioglu D, Lopes JM, Kleihues P, Ohgaki H. Genetic profile of gliosarcomas. Am J Pathol. 2000. 156: 425-32
41. Salunke P, Singh H, Vaiphei K. Lateral ventricular gliosarcoma with attachment to septum pellucidum. Asian J Neurosurg. 2017. 12: 82-4
42. Sampaio L, Linhares P, Fonseca J. Detailed magnetic resonance imaging features of a case series of primary gliosarcoma. Neuroradiol J. 2017. 30: 546-53
43. Sarkar H, K S, Ghosh S. Pure intraventricular origin of gliosarcoma-a rare entity. Turk Neurosurg. 2013. 23: 392-4
44. Singh G, Mallick S, Sharma V, Joshi N, Purkait S, Jha P. A study of clinico-pathological parameters and O6-methylguanine DNA methyltransferase (MGMT) promoter methylation status in the prognostication of gliosarcoma. Neuropathology. 2012. 32: 534-42
45. Smith DR, Wu CC, Saadatmand HJ, Isaacson SR, Cheng SK, Sisti MB. Clinical and molecular characteristics of gliosarcoma and modern prognostic significance relative to conventional glioblastoma. J Neurooncol. 2018. 137: 303-11
46. Witwer BP, Salamat MS, Resnick DK. Gliosarcoma metastatic to the cervical spinal cord: Case report and review of the literature. Surg Neurol. 2000. 54: 373-9
47. Wong E, Nahar N, Hau E, Varikatt W, Gebski V, Ng T. Cut-point for Ki-67 proliferation index as a prognostic marker for glioblastoma. Asia Pac J Clin Oncol. 2019. 15: 5-9
48. Zhang G, Huang S, Zhang J, Wu Z, Lin S, Wang Y. Clinical outcome of gliosarcoma compared with glioblastoma multiforme: A clinical study in Chinese patients. J Neurooncol. 2016. 127: 355-62