- Department of Neurosurgery Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan.
- Department of Neurosurgery, Harasanshin Hospital, Fukuoka, Japan.
- Department of Pediatrics, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan.
- Department of Clinical Chemistry and Laboratory, Kyushu University Hospital, Fukuoka, Japan.
- Department of Psychiatry, Shourai Hospital, Karatsu, Japan.
Correspondence Address:
Nobutaka Mukae
Department of Neurosurgery Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan.
DOI:10.25259/SNI_28_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Nobutaka Mukae1, Takato Morioka2, Michiko Torio3, Yasunari Sakai3, Takafumi Shimogawa1, Ayumi Sakata4, Satoshi O. Suzuki5, Masahiro Mizoguchi1. Periodic discharges with high frequency oscillations recorded from a cerebellar gangliocytoma in an epileptic infant. 17-Mar-2021;12:98
How to cite this URL: Nobutaka Mukae1, Takato Morioka2, Michiko Torio3, Yasunari Sakai3, Takafumi Shimogawa1, Ayumi Sakata4, Satoshi O. Suzuki5, Masahiro Mizoguchi1. Periodic discharges with high frequency oscillations recorded from a cerebellar gangliocytoma in an epileptic infant. 17-Mar-2021;12:98. Available from: https://surgicalneurologyint.com/surgicalint-articles/10653/
Abstract
Background: Subcortical epilepsies associated with developmental tumors in the cerebellum are rarely experienced. As supportive evidence of the intrinsic epileptogenicity of cerebellar tumors, previous electroencephalogram (EEG) studies with intratumoral depth electrodes demonstrated epileptiform or ictal discharges. Recent studies have demonstrated that high frequency oscillations (HFOs) can be regarded as a new biomarker of epileptogenesis and ictogenesis; however, there are few evidence about HFOs in cases of epilepsy associated with cerebellar tumors.
Case Description: A 6-month-old Japanese male infant presented to our hospital with drug resistant epilepsy. We underwent subtotal resection of a cerebellar gangliocytoma and obtained good seizure outcomes. Intraoperative EEG in the tumor depicted HFOs in the form of ripples, riding on periodic discharges.
Conclusion: Our findings provide further supportive evidence for the intrinsic epileptogenicity of cerebellar tumors.
Keywords: Cerebellar epilepsy, Depth electrode, High frequency oscillations, Intrinsic epileptogenicity, Subcortical epilepsy
INTRODUCTION
Classical teaching in epileptology localizes the origin of focal seizures solely in the cerebral cortex; however, recent electrophysiological and neuroimaging studies have provided evidence for the initiation of epileptic seizures within subcortical structures.[
High frequency oscillations (HFOs) are a form of brain activity observed in EEG in the frequency range of 80–500 Hz. HFOs can be classified into ripples (80–200 Hz) and fast ripples (200–500 Hz) on the basis of their distinctive characteristics. Recent studies have reported that both ripples and fast ripples can be regarded as new biomarkers of epileptogenesis and ictogenesis.[
CASE REPORT
This male infant was diagnosed with mild ventriculomegaly on prenatal ultrasound examination. He was born at 39 weeks and 1 day of gestation with an uneventful delivery. Magnetic resonance (MR) images at the age of 3 days revealed a tumor in the right cerebellum protruding into the 4th ventricle. Conservative follow-up was selected because he was asymptomatic. However, 2 weeks after birth, he developed epileptic seizures starting with horizontal nystagmus and conjugate deviation to the right side, followed by tonic-clonic seizures of his left upper limb. His seizure frequency was 4–5 times/h. Optimal doses of antiepileptic drugs including phenobarbital, levetiracetam, perampanel, and topiramate were administered. His seizure frequency was reduced to 1–2 times/h; however, complete control could not be obtained.
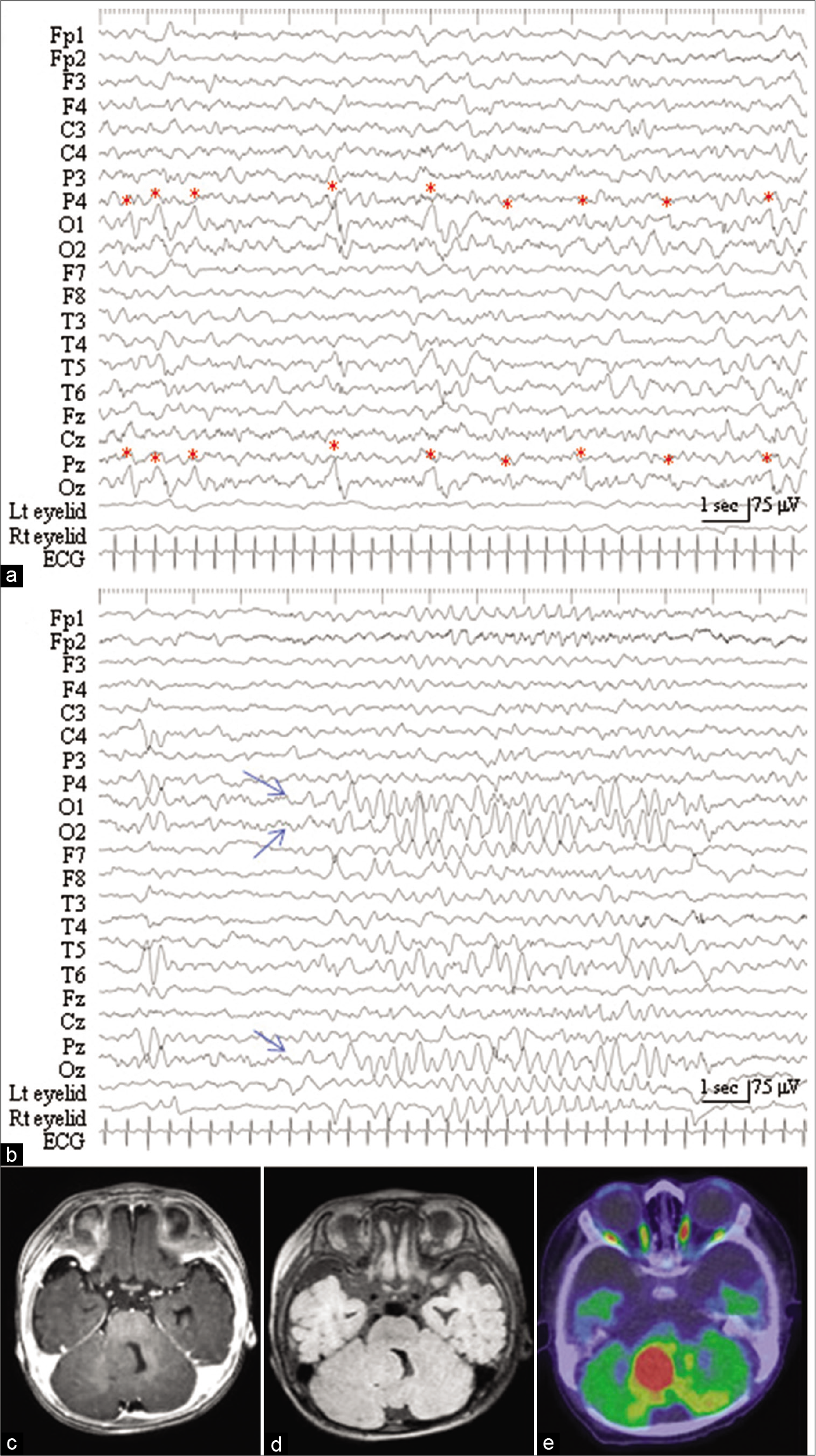
The patient was referred to us at the age of 3 months. His height and body weight were 65.4 cm and 7.1 kg, respectively, which were age-appropriate. During the interictal state, his neurological findings were normal. His seizure began with the twitching of the bilateral eyelid, horizontal nystagmus, and ocular displacement lasting 10 s to 1 min and was sometimes followed by tonic seizures in his right upper and lower limbs. Interictal EEG revealed periodic discharges with a predominant negative component occurring once every 1–4 s in the left and mid-occipital regions (O1 and Oz of the International EEG 10–20 system, respectively) [
Figure 1:
(a) Interictal electroencephalogram (EEG), with an averaged reference, reveals periodic discharges with a predominant negative component occurring once every 1–4 s in the left and mid-occipital region (O1 and Oz of the International EEG 10–20 system, respectively, red asterisks). (b) Ictal EEG, with the twitching of bilateral eyelids, demonstrates rhythmic slow waves originating from the occipital region (O1, O2, and Oz, blue arrows). (c) Preoperative axial-view of T1-weighted (Gd) administration shows an isointensity tumor in the right cerebellum protruding into the 4th ventricle. No Gd enhancement was noted. (d) The tumor is presented in an isointense on preoperative axial view of image with fluid level attenuated inversion recovery sequences. (e) Fusion image of 18F-fluorodeoxyglucose positron emission tomography and computed tomographic scan, at the level comparable to the panel (c), depicts hypermetabolism of the tumor.
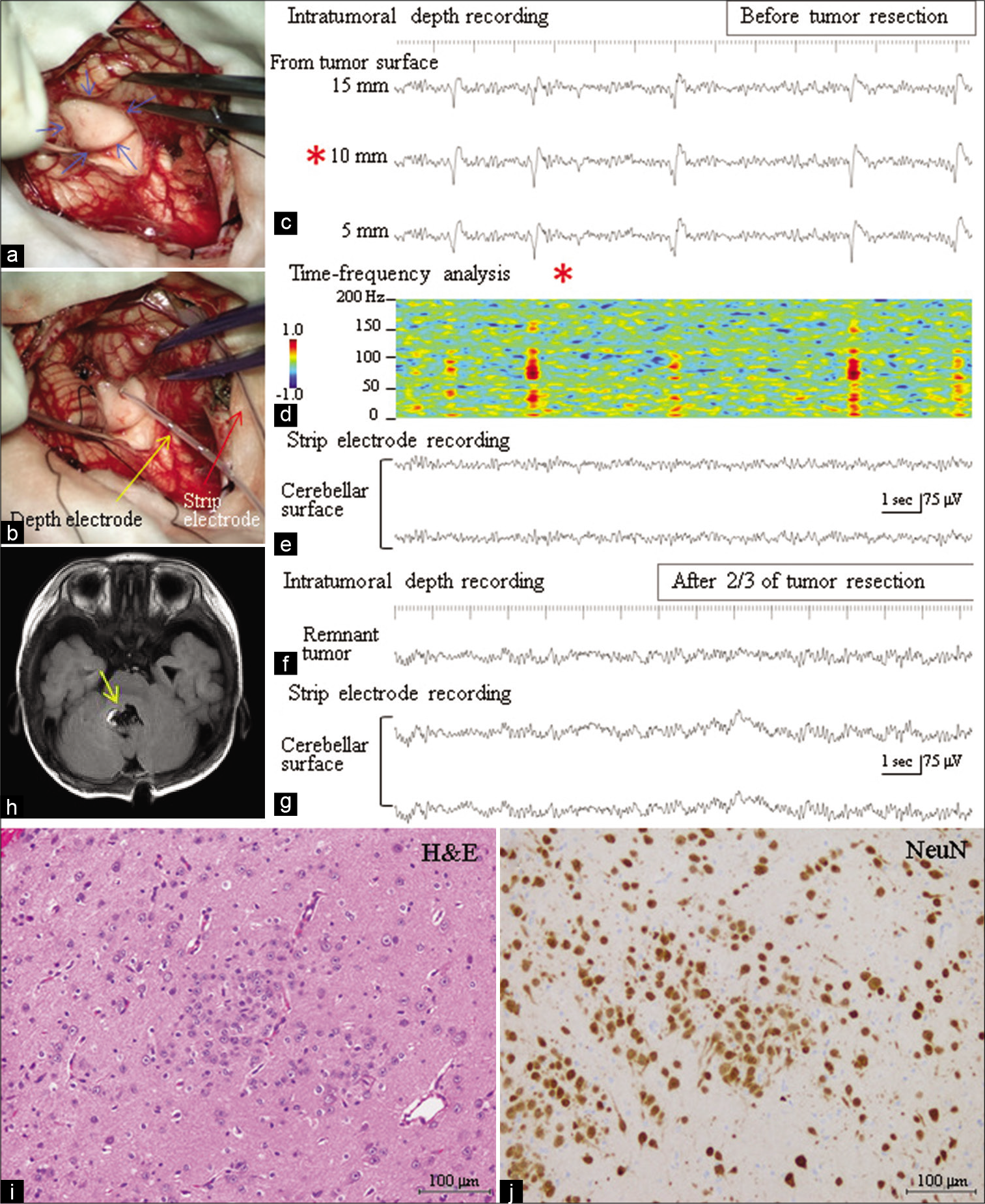
At 6 months of age, surgery was performed under general propofol anesthesia. Through the trans-cerebellomedullary fissure approach, a slightly whitish tumor protruding into the 4th ventricle was exposed [
Figure 2:
(a) Intraoperative photograph shows that a tumor (blue arrows) protruding into the 4th ventricle is exposed through the transcerebellomedullary fissure approach. (b) A depth electrode with 3 contacts (yellow arrow) is inserted into the tumor, and a strip electrode (red arrow) is placed on the cerebellar surface. (c) Intraoperative depth electroencephalogram (EEG) recording from the tumor, with A1 reference, depicts periodic discharges with a positive-negative configuration occurring once every 1–4 s. The periodic discharges show maximum amplitude at a depth of 10 mm from the tumor surface (red asterisk), with a slight decrease in amplitude at 5 and 15 mm depths. (d) Time-frequency analysis (short-term fast Fourier transformation) for the EEG at a depth of 10 mm from the tumor surface (red asterisk) demonstrates high frequency oscillatory activity around 80–100 Hz (ripple range), riding on the periodic discharges. Color coordinates were constructed on a logarithmic scale (–1.0 to 1.0). (e) No paroxysmal discharge was recorded from the cerebellar surface. (f) Following the resection of three-quarters of the tumor, the tip of the depth electrode was inserted again into the remnant tumor. EEG demonstrates the disappearance of paroxysmal activity from the remnant tumor. (g) No paroxysmal discharge was noted on the cerebellar surface as was observed before resection of the tumor. (h) Postoperative T1-weighted magnetic resonance image demonstrates that the tumor was subtotally resected except for the tumor around the right facial colliculus (yellow arrow). (i and j) Histopathological findings of the tumor with hematoxylin and eosin staining (i) and immunostaining for neuronal nuclei (NeuN) (j). The tumor consists of irregular clustering of NeuN-immunopositive ganglion cells without a glioma component.
The postoperative course was uneventful. The patient was seizure-free within 2 years following the operation, even after reduced dosages of antiepileptic drugs. Postoperative EEG demonstrated the disappearance of paroxysmal activity. Histopathologically, the tumor consisted of irregularly clustered neuronal nuclei (NeuN) immunopositive ganglion cells without a glioma component, indicating gangliocytoma [
DISCUSSION
Previous authors have demonstrated, with the use of perioperative depth recording, the existence of ictal discharges originating from a tumor[
Recent studies reported that interictal HFOs can be regarded as a new biomarker of epileptogenesis and ictogenesis.[
Another notable finding was that the periodic discharges with HFOs showed maximum amplitude in the center of the tumor, with a slight decrease in amplitude at the periphery, and were not recorded in the remnant tumor after three-quarters of the tumor was resected. These findings indicate that a tumor of a certain size is needed to cause epileptic activity and support the findings that the subtotal or partial removal of the tumor can cease epileptic seizures.[
Although our experience is limited to a single case, the finding adds further supportive evidence of intrinsic epileptogenicity of the cerebellar tumor.
Statement of ethics
This research was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. Written informed consent was obtained from the patient’s parents for publication of this case report and any accompanying images.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Boop S, Wheless J, van Poppel K, McGregor A, Boop FA. Cerebellar seizures. J Neurosurg Pediatr. 2013. 12: 288-92
2. Chae JH, Kim SK, Wang KC, Kim KJ, Hwang YS, Cho BK. Hemifacial seizure of cerebellar ganglioglioma origin: Seizure control by tumor resection. Epilepsia. 2001. 42: 1204-7
3. Cho JR, Joo EY, Koo DL, Hong SC, Hong SB. Clinical utility of interictal high-frequency oscillations recorded with subdural macroelectrodes in partial epilepsy. J Clin Neurol. 2012. 8: 22-34
4. Cimbalnik J, Kucewicz MT, Worrell G. Interictal high-frequency oscillations in focal human epilepsy. Curr Opin Neurol. 2016. 29: 175-81
5. Delande O, Rodriguez D, Chiron C, Fohlen M. Successful surgical relief of seizures associated with hamartoma of the floor of the fourth ventricle in children: Report of two cases. Neurosurgery. 2001. 49: 726-30
6. Foit NA, van Velthoven V, Schulz R, Blcke I, Urbach H, Woermann FG. Lesional cerebellar epilepsy: A review of the evidence. J Neurol. 2017. 264: 1-10
7. Frauscher B, von Ellenrieder N, Zelmann R, Rogers C, Nguyen DK, Kahane P. High-frequency oscillations in the normal human brain. Ann Neurol. 2018. 84: 374-85
8. Harvey AS, Jayakar P, Duchowny M, Resnick T, Prats A, Altman N. Hemifacial seizures and cerebellar ganglioglioma: An epilepsy syndrome of infancy with seizures of cerebellar origin. Ann Neurol. 1996. 40: 91-8
9. Koh KN, Lim BC, Hwang H, Park JD, Chae JH, Kim KJ. Cerebellum can be a possible generator of progressive myoclonus. J Child Neurol. 2010. 25: 728-31
10. Kulkarni S, Hegde A, Shah KN. Hemifacial seizures and cerebellar tumor: A rare co-existence. Indian Pediatr. 2007. 44: 378-9
11. Kuzniecky R, Guthrie B, Mountz J, Bebin M, Faught E, Gilliam F. Intrinsic epileptogenesis of hypothalamic hamartomas in gelastic epilepsy. Ann Neurol. 1997. 42: 60-7
12. Lascano AM, Lemkaddem A, Granziera C, Korff CM, Boex C, Jenny B. Tracking the source of cerebellar epilepsy: Hemifacial seizures associated with cerebellar cortical dysplasia. Epilepsy Res. 2013. 105: 245-9
13. Martins WA, Paglioli E, Hemb M, Palmini A. Dysplastic cerebellar epilepsy: Complete seizure control following resection of a ganglioglioma. Cerebellum. 2016. 15: 535-41
14. Mesiwala AH, Kuratani JD, Avellino AM, Roberts TS, Sotero MA, Ellenbogen RG. Focal motor seizures with secondary generalization arising in the cerebellum. Case report and review of the literature. J Neurosurg. 2002. 97: 190-6
15. Nishio S, Morioka T, Fukui M, Goto Y. Surgical treatment of intractable seizures due to hypothalamic hamartoma. Epilepsia. 1994. 35: 514-9
16. Park CJ, Hong SB. High frequency oscillations in epilepsy: Detection methods and considerations in clinical application. J Epilepsy Res. 2019. 9: 1-13
17. Park YS, Oh MC, Kim HD, Kim DS. Early surgery of hamartoma of the floor of the fourth ventricle: A case report. Brain Dev. 2009. 31: 347-51
18. Worrell GA, Parish L, Cranstoun SD, Jonas R, Baltuch G, Litt B. High-frequency oscillations and seizure generation in neocortical epilepsy. Brain. 2004. 127: 1496-506
19. Yagyu K, Sueda K, Shiraishi H, Asahina N, Sakurai K, Kohsaka S. Direct correlation between the facial nerve nucleus and hemifacial seizures associated with a gangliocytoma of the floor of the fourth ventricle: A case report. Epilepsia. 2011. 52: e204-6