- Department of Neurosurgery, University of Alberta, King Faisal University, Office of Postgraduate Surgical Education, University of Alberta, Edmonton, Canada
- Department of Neurosurgery, University of Alberta, Office of Postgraduate Surgical Education, University of Alberta, Edmonton, Canada
- Department of Neurosurgery, University of Alberta, King Fahad Medical City, Office of Postgraduate Surgical Education, University of Alberta, Edmonton, Canada
- Department of Neuropathology, University of Alberta, Walter MacKenzie Health Sciences Centre, Edmonton, Canada.
Correspondence Address:
Abdullah Alarfaj, Department of Neurosurgery, University of Alberta, King Faisal University, Office of Postgraduate Surgical Education, Edmonton, Canada.
DOI:10.25259/SNI_465_2023
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Abdullah Alarfaj1, Brooke Pollock2, Abdelaziz Sagga3, Sumit Das4, Michael Chow2. Astrocytoma with high-grade features and MYBL1-MMP16 fusion. 21-Jun-2024;15:203
How to cite this URL: Abdullah Alarfaj1, Brooke Pollock2, Abdelaziz Sagga3, Sumit Das4, Michael Chow2. Astrocytoma with high-grade features and MYBL1-MMP16 fusion. 21-Jun-2024;15:203. Available from: https://surgicalneurologyint.com/surgicalint-articles/12959/
Abstract
Background: Gliomas represent the most common primary intraparenchymal brain tumors in adult and pediatric patients. Neuropathological work-up of these gliomas typically entails the determination of isocitrate dehydrogenase (IDH) mutational status, presence or absence of 1p/19q co-deletion, and O6 methylguanine-DNA methyl-transferase (MGMT) promoter methylation status.
Case Description: We present here an unusual case of a posterior fossa tumor in a 51-year-old female, which was initially diagnosed as astrocytoma with some high-grade features that recurred, displaying even more aggressive features such as infiltration and increased proliferative activity. Both the initially resected and recurrent tumor revealed MYBL1-MMP16 fusion, which is much more commonly found in pediatric low-grade gliomas and, to our knowledge has not been described in the context of an adult glioma.
Conclusion: The significance of MYBL1-MMP16 fusion in adult gliomas in relation to survival and likelihood of recurrence is, therefore, unknown and requires more extensive research.
Keywords: High-grade astrocytoma, Low-grade astrocytoma, MYBL1-MMP16 fusion
INTRODUCTION
Gliomas are the most common primary tumors of the central nervous system (CNS), with astrocytomas and oligodendrogliomas as the most common morphological subtypes.[
The authors here report an unusual case of a posterior fossa tumor in a 51-year-old female who was initially diagnosed with astrocytoma with some high-grade features that recurred, displaying even more aggressive features; this case is unusual in that both the initial and recurrent tumor revealed a MYBL1-MMP16 fusion, which, to our knowledge, has been previously described in the context of pediatric low-grade gliomas,[
CASE
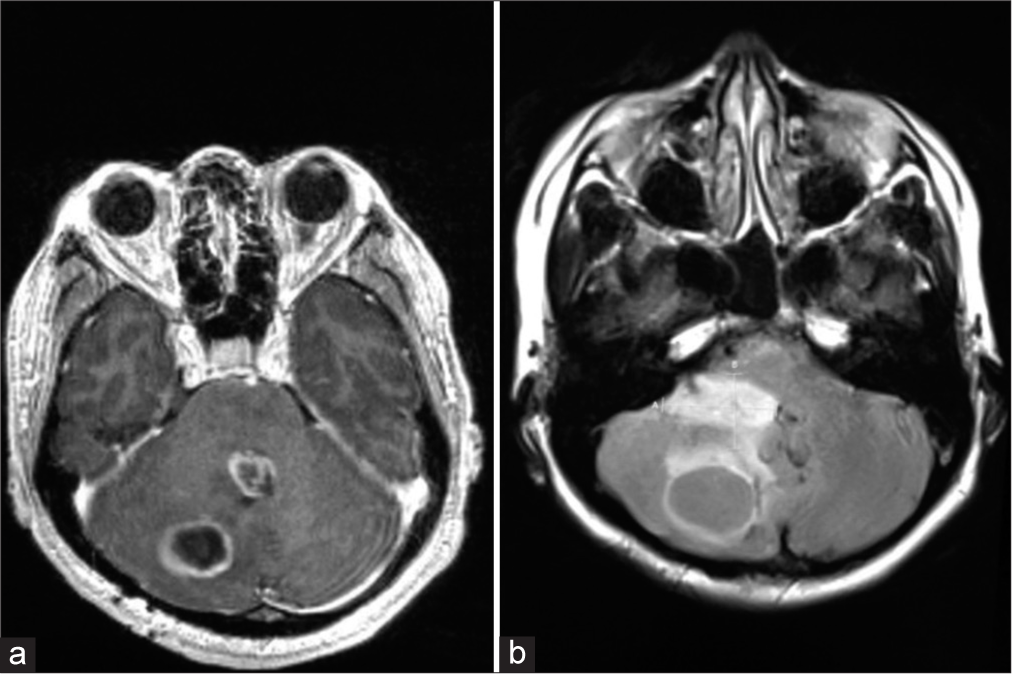
Our patient is a 51-year-old female who initially presented with vertigo and, over two weeks, developed nausea, vomiting, headache, ataxia, and diplopia. On examination, she was found to have bilateral papilledema and mild right-sided dysdiadochokinesia. Initial investigations included computed tomography (CT) and magnetic resonance imaging (MRI) of the head, which revealed a cystic mass of the right cerebellar hemisphere measuring 4.1 × 3.2 × 3.2 cm [
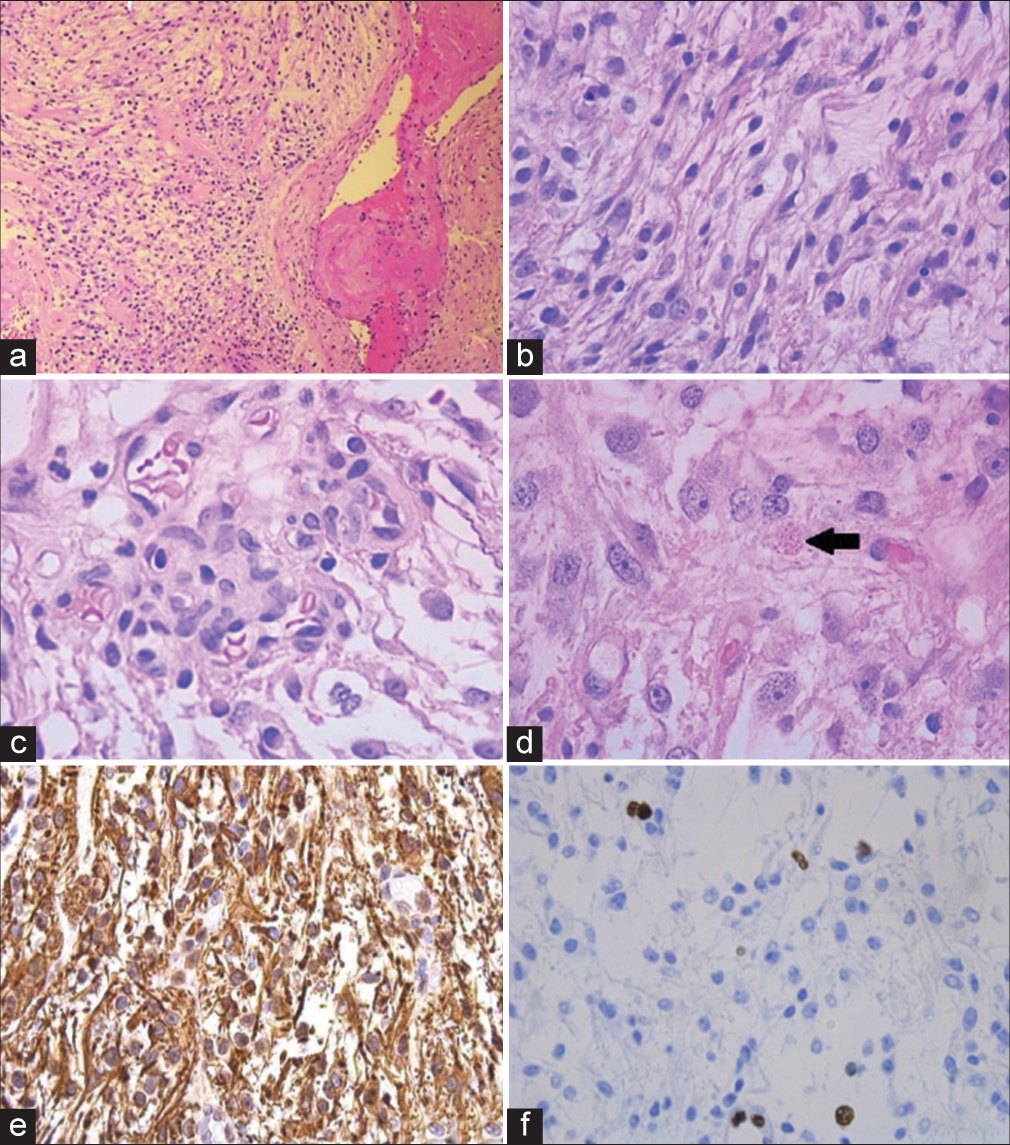
Neuropathological examination of the resected tumor revealed a glial tumor comprised of Glial fibrillary acidic protein (GFAP)-positive astrocytic cells, some of which appeared to show fibrillary cytoplasmic processes. Rare examples of eosinophilic granular bodies and Rosenthal fibers were also found. Mitotic figures were scarce, and MIB-1/Ki67 was estimated at 5%. Vascular proliferations were also seen. In light of these findings, the tumor was histologically felt to be consistent with diffuse astrocytoma with high-grade features, as shown in
Figure 2:
(a) Low magnification view of the tumor with a thrombosed blood vessel; (b) higher magnification showing glial cells with piloid processes; (c) example of microvascular proliferation; (d) example of eosinophilic granular body (arrow); (e) glial fibrillary acidic protein (GFAP) immunohistochemistry showing diffuse immunoreactivity; and (f) low Ki67/MIB-1 proliferative index.
A postoperative MRI done four months after surgery showed a small infiltrative residual tumor behaving like a diffuse glioma [
Figure 4:
Eleven months after the first surgery, the patient presented with tumor recurrence. (a) Two lesions, one in the right cerebellar hemisphere, which is consistent with the location of the original tumor. Another lesion is seen centered in the fourth ventricle, likely arising from the residual tumor infiltrating the ventricular wall; (b) axial flair magnetic resonance imaging shows a portion of the tumor arising from the right lateral aspect of the fourth ventricular wall.
Unfortunately, methylation profiling was not available at the time of reporting. The patient traveled out of the country and was lost in follow-up.
DISCUSSION
We present here an unusual case of a posterior fossa tumor in a 51-year-old female, which was initially diagnosed as astrocytoma with some high-grade features that recurred, displaying even more aggressive features. What is unusual about our patient is that although the recurrent tumor revealed aggressive features (infiltration, frequent mitotic activity, and elevated proliferative index), molecular analysis still revealed a MYBL1-MMP16 fusion, which is more in keeping with an indolent glioma and no additional mutations on top of those found in the initial tumor were detected. The majority of those gliomas seem to occur in pediatric patients and young adults, while presentations in older adults (>50 years) are much less common.[
Early-described mutations in astrocytomas, such as PAs, include mutations to PDGF ligands and PDGF receptors, as well as inactivating mutations to TP53.[
CONCLUSION
Herein, the authors present an unusual case of a recurrent astrocytic tumor with histopathological features compatible with an astrocytoma with high-grade features. The MYBLMMP16 fusion found in both the initial and recurrent tumor has been reported in pediatric low-grade gliomas, but its significance in adult gliomas is unclear and requires further investigation. Larger scale research is necessary to determine their clinical significance and potential prognostic and therapeutic implications. Cases such as ours also reiterate the notion that neuropathologic diagnosis of CNS tumors must be a true integration of histopathological features and molecular data rather than the misconception that diagnostic and management decisions can be made solely based on molecular data. Further, an understanding of molecular features may provide insight into the more aggressive nature of these tumors in this population.
Ethical approval
Institutional review board approval is not required.
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
Acknowledgments
The authors would like thanks to Dr. C. Hawkins for performing the molecular testing for this case.
References
1. Azad A, Deb S, Cher L. Primary anaplastic pilocytic astrocytoma. J Clin Neurosci. 2009. 16: 1704-6
2. Bandopadhayay P, Ramkissoon LA, Jain P, Bergthold G, Wala J, Zeid R. MYB-QKI rearrangements in angiocentric glioma drive tumorigenicity through a tripartite mechanism. Nat Genet. 2016. 48: 273-82
3. Behin A, Hoang-Xuan K, Carpentier AF, Delattre JY. Primary brain tumours in adults. Lancet. 2003. 361: 323-31
4. Dolecek TA, Propp JM, Stroup NE, Kruchko C. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neuro Oncol. 2012. 14: v1-49
5. Ellison DW, Hawkins C, Jones DT, Onar-Thomas A, Pfister SM, Reifenberger G. cIMPACT-NOW update 4: Diffuse gliomas characterized by MYB, MYBL1, or FGFR1 alterations or BRAF(V600E) mutation. Acta Neuropathol. 2019. 137: 683-7
6. Forshew T, Tatevossian RG, Lawson AR, Ma J, Neale G, Ogunkolade BW. Activation of the ERK/MAPK pathway: A signature genetic defect in posterior fossa pilocytic astrocytomas. J Pathol. 2009. 218: 172-81
7. Johnson DR, Brown PD, Galanis E, Hammack JE. Pilocytic astrocytoma survival in adults: analysis of the surveillance, epidemiology, and end results program of the national cancer institute. J Neurooncol. 2012. 108: 187-93
8. Koeller KK, Rushing EJ. From the archives of the AFIP: Pilocytic astrocytoma: Radiologic-pathologic correlation. Radiographics. 2004. 24: 1693-708
9. Lee KJ, Marchan E, Peterson J, Harrell AC, Quinones-Hinojosa A, Brown PD. Management and survival of adult patients with pilocytic astrocytoma in the national cancer database. World Neurosurg. 2018. 112: 881-7
10. Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK. The 2016 world health organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016. 131: 803-20
11. Ohgaki H, Kleihues P. Population-based studies on incidence, survival rates, and genetic alterations in astrocytic and oligodendroglial gliomas. J Neuropathol Exp Neurol. 2005. 64: 479-89
12. Qaddoumi I, Orisme W, Wen J, Santiago T, Gupta K, Dalton JD. Genetic alterations in uncommon low-grade neuroepithelial tumors: BRAF, FGFR1, and MYB mutations occur at high frequency and align with morphology. Acta Neuropathol. 2016. 131: 833-45
13. Ramkissoon LA, Horowitz PM, Craig JM, Ramkissoon SH, Rich BE, Schumacher SE. Genomic analysis of diffuse pediatric low-grade gliomas identifies recurrent oncogenic truncating rearrangements in the transcription factor MYBL1. Proc Natl Acad Sci U S A. 2013. 110: 8188-93
14. Rodriguez FJ, Vizcaino MA, Lin MT. Recent advances on the molecular pathology of glial neoplasms in children and adults. J Mol Diagn. 2016. 18: 620-34
15. Shibahara I, Kawaguchi T, Kanamori M, Yonezawa S, Takazawa H, Asano K. Pilocytic astrocytoma with histological malignant features without previous radiation therapy--case report. Neurol Med Chir (Tokyo). 2011. 51: 144-7
16. Stüer C, Vilz B, Majores M, Becker A, Schramm J, Simon M. Frequent recurrence and progression in pilocytic astrocytoma in adults. Cancer. 2007. 110: 2799-808
17. Theeler BJ, Ellezam B, Sadighi ZS, Mehta V, Tran MD, Adesina AM. Adult pilocytic astrocytomas: Clinical features and molecular analysis. Neuro Oncol. 2014. 16: 841-7
18. Thome I, Lacle R, Voß A, Bortolussi G, Pantazis G, Schmidt A. Neoplastic cells are the major source of MT-MMPs in IDH1-mutant glioma, thus enhancing tumor-cell intrinsic brain infiltration. Cancers (Basel). 2020. 12: 2456
19. Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009. 360: 765-73
20. Ye JM, Ye MJ, Kranz S, Lo P. A 10 year retrospective study of surgical outcomes of adult intracranial pilocytic astrocytoma. J Clin Neurosci. 2014. 21: 2160-4
21. Yong VW, Power C, Forsyth P, Edwards DR. Metalloproteinases in biology and pathology of the nervous system. Nat Rev Neurosci. 2001. 2: 502-11