- Department of Stem Cell Biology, School of Medicine, Konkuk University, Seoul, Korea
- Department of Laboratory Animal Medicine, College of Veterinary Medicine, Seoul National University, Seoul, Korea
Correspondence Address:
C-Yoon Kim
Department of Stem Cell Biology, School of Medicine, Konkuk University, Seoul, Korea
DOI:10.4103/2152-7806.190474
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Kim C, Oh H, Hwang I, Hong K. GEMINI: Initial behavioral results after full severance of the cervical spinal cord in mice. Surg Neurol Int 13-Sep-2016;7:
How to cite this URL: Kim C, Oh H, Hwang I, Hong K. GEMINI: Initial behavioral results after full severance of the cervical spinal cord in mice. Surg Neurol Int 13-Sep-2016;7:. Available from: http://surgicalneurologyint.com/surgicalint_articles/gemini-initial-behavioral-results-full-severance-cervical-spinal-cord-mice/
Abstract
Background:The GEMINI spinal cord fusion protocol has been developed to achieve a successful cephalosomatic anastomosis. Here, we report the preliminary data on the use of a fusogen [polyethylene glycol (PEG)] after full cervical cord transection in mice to facilitate the fusion of both ends of a sharply transected spinal cord.
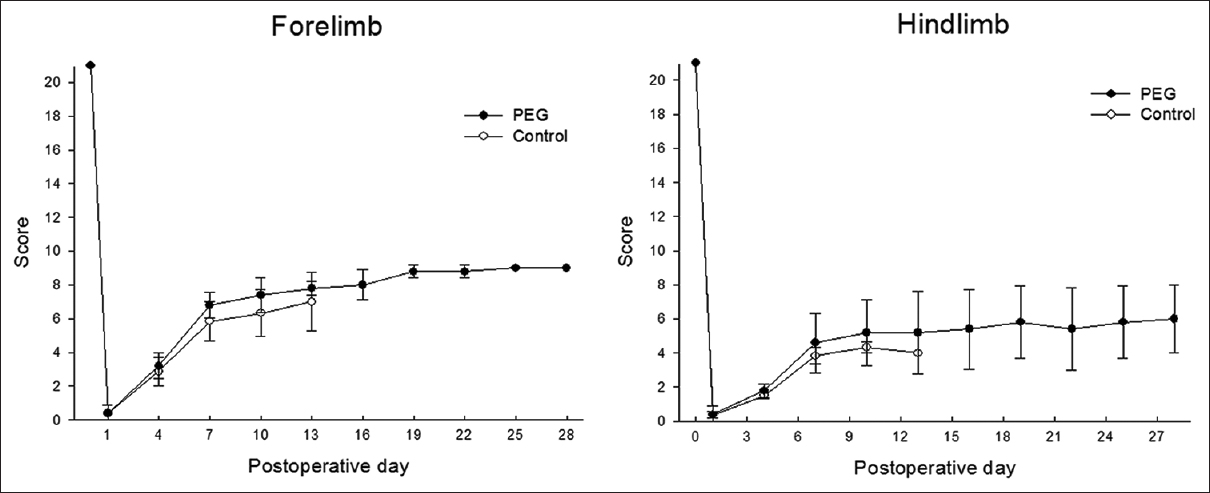
Methods:Cervical laminectomy and a complete, visually confirmed cervical cord (C 5) transection was performed on female albino mice (n = 16). In Group 1 (n = 8), a fusogen, (PEG) was used to bridge the gap between the cut ends of the spinal cord. Group 2 received the same spinal cord transection but was treated with saline. Outcome was assessed daily using a standard scale (modified 22-point Basso-Beattie-Bresnahan scale) and filmed on camera.
Results:The PEG group (group 1) showed partial restoration of motor function after 4 weeks of observation; group 2 (placebo) did not recover any useful motor activity.
Conclusion:In this preliminary experiment, PEG, but not saline, promoted partial motor recovery in mice submitted to full cervical transection.
Keywords: Cephalosomatic anastomosis, GEMINI, PEG, spinal cord fusion
INTRODUCTION
To achieve a successful spinal cord fusion as required during cephalosomatic anastomosis (CSA), an effective technique to assure rapid reinnervation of the body across the divided segments of the cervical spinal cord is necessary.[
This fusion of transected axons, as demonstrated experimentally (reviewed in[
We recently reported the effects of PEG applied to the severed cervical cord with initial recovery of motor evoked potentials at 1 hour in rats.[
MATERIALS AND METHODS
The experiment was carried out in accordance with the Animal Ethics Committees guidelines and was approved by the Institutional Animal Care and Use Committee of Konkuk University (Seoul, South Korea).
Surgery
Female albino (ICR: Imprinting control region) mice (25~30 g, n = 16) were anesthetized using zoletil and xylazine (3:1 ratio, 1 ml/kg). Cervical laminectomy was performed, and the muscles overlying the vertebral column reflected exposing C4-6 laminae and spinous processes; the C5 spinous process was carefully removed. After gently raising the cervical cord with a hook that atraumatically coursed all around the cord, severance was performed with surgical sharp blades #11 in two passes to visually confirm transection. PEG (PEG MW 400, Sigma-Aldrich, USA) (Group 1, n = 8) or phosphate buffered saline (PBS) (Group 2, n = 8) were applied to the cut area.
The muscle and fascia were sutured and the skin closed. Dextrose 5% solution (20 ml/kg) was administered daily via intraperitoneal injection. After consciousness was regained, breathing and heart rate were maintained autonomously, and body temperature was maintained at a constant level (27–29°C) in an incubator. To prevent dehydration, normal saline solution was provided with total parenteral nutrition (TPN, Chong Kun Dang, Korea) through the tail vein and abdominal cavity 4 times a day.
Functional assessment
The modified 22-point Basso-Beattie-Bresnahan (mBBB) Locomotor Rating Scale,[
RESULTS
Functional recovery
After 24 hours, very slight movements of the forelimbs were observed in all 8 treated animals [
DISCUSSION
In this initial study, we tested one arm of the proposed GEMINI spinal cord fusion protocol, namely the use of fusogens applied topically to the sharply cervical severed spinal cord. We reported on the ultra-early results of PEG application,[
In this behavioral study, histological analysis is not reported (manuscript in preparation), although preliminary analysis suggests that fibers regrew, local limited scarring notwithstanding. This is in line with the recent data that show that following spinal cord injury the astrocytic scar aids, rather than inhibits, axon regeneration, in rodents (the reader is referred to discussion in reference 5 for more details). This result[
The death of rats after cervical spinal cord transection is not unexpected, and is likely due to cachexia following intestinal functional disturbance, as assessed at autopsy.
One deficiency of this work is that controls died before the end of the study. As Freeman observed, if given enough time, animals submitted to spinal transection will recover. In our study, controls also showed an initial recovery at 2 weeks, but of a lesser degree than PEG-treated rats. No statistical analysis was conducted due to the small sample; also we could not compare rats at equal time-points. However, the data is already strong to confirm Freeman's observations, that a sharp section is not associated with permanent impairment.
In sum, in this preliminary set of data, we show that a severed cervical spinal cord does not lead to permanent motor impairment in mice if treated with GEMINI spinal cord fusogens, in agreement with other data at dorsal level.[
Financial support and sponsorship
The research was partially supported by the National Research Foundation of Korea (NRF) Grant (2015R1C1A1A02037047).
Conflicts of interest
There are no conflicts of interest.
Video is Availabe on: www.surgicalneurologyint.com
Acknowledgement
We thank Prof Canavero for assistance in writing the paper and assisting with the interpretation of the data.
References
1. Brazda N, Estrada V, Voss C, Seide K, Trieu HK, Müller HW. Experimental Strategies to Bridge Large Tissue Gaps in the Injured Spinal Cord after Acute and Chronic Lesion. J Vis Exp. 2016. 110: e53331-
2. Canavero S. HEAVEN: The head anastomosis venture project outline for the first human head transplantation with spinal linkage (GEMINI). Surg Neurol Int. 2013. 4: S335-42
3. Canavero S, Ren XP, Kim CY, Rosati E. Neurologic foundations of spinal cord fusion (GEMINI). Surgery. 2016. 160: 11-9
4. Fenrich KK, Rose PK. Spinal interneuron axons spontaneously regenerate after spinal cord injury in the adult feline. J Neurosci. 2009. 29: 12145-58
5. Kim CY. PEG-assisted reconstruction of the cervical spinal cord in rat: Effects on motor conduction at 1 hour. Spinal Cord (Article in Press). 2016. p.
6. Liddelow SA, Barres BA. Regeneration: Not everything is scary about a glial scar. Nature. 2016. 532: 182-3
7. Martinez M, Brezun JM, Bonnier L, Xerri C. A new rating scale for open-field evaluation of behavioral recovery after cervical spinal cord injury in rats. J Neurotrauma. 2009. 26: 10143-53
8. Ye YJ, Kim CY, Miao Q, Ren XP. Fusogen-assisted rapid reconstitution of anatomophysiologic continuity of the transected spinal cord. Surgery. 2016. 160: 20-5







Luis Manuel Muegues Acosta
Posted November 19, 2017, 5:07 pm
Hello,
I have a question. My name is Luis and I have been studying neuroscience since 2007. I understand the proposed protocol for healing the connections of the spinal cord. But after connection, the same “cables” will not be connected with the same “cable nerve” so that the electrical signal will now, in case it get reconnected again, not be sent to the same nerve. This will have as consequence that one electrical signal from the brain will be send elsewhere in most of the cases. Does this mean that the brain, will need to relearn how to regulate (and command) the entire body? Including longs, and organs? How is it possible that the maize could start breathing? I know that because of neuroplasticity, the brain will be able to relearn how to command this new path ways but how can this happen so fast!