- Department of Neurosurgery, University of Pittsburgh, Pittsburgh, Pennsylvania, United States,
- Department of Neurosurgery, Neurosurgery Teaching Hospital, Al Risafa, Baghdad, Iraq,
- Department of Neurology, University of Pittsburgh Medical Center Stroke Institute, Pittsburgh, Pennsylvania, United States.
Correspondence Address:
Samer S. Hoz, Department of Neurosurgery, University of Pittsburgh, Pittsburgh, Pennsylvania, United States.
DOI:10.25259/SNI_379_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Samer S. Hoz1, Li Ma1, Mustafa Ismail2, Alhamza R. Al-Bayati3, Raul G. Nogueira3, Michael J. Lang1, Bradley A. Gross1. Intracranial aneurysms and abducent nerve palsy. 21-Jun-2024;15:207
How to cite this URL: Samer S. Hoz1, Li Ma1, Mustafa Ismail2, Alhamza R. Al-Bayati3, Raul G. Nogueira3, Michael J. Lang1, Bradley A. Gross1. Intracranial aneurysms and abducent nerve palsy. 21-Jun-2024;15:207. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=12955
Abstract
Background: Cranial nerve (CN) palsy may manifest as an initial presentation of intracranial aneurysms or due to the treatment. The literature reveals a paucity of studies addressing the involvement of the 6th CN in the presentation of cerebral aneurysms.
Methods: Clinical patient data, aneurysmal characteristics, and CN 6th palsy outcome were retrospectively reviewed and analyzed.
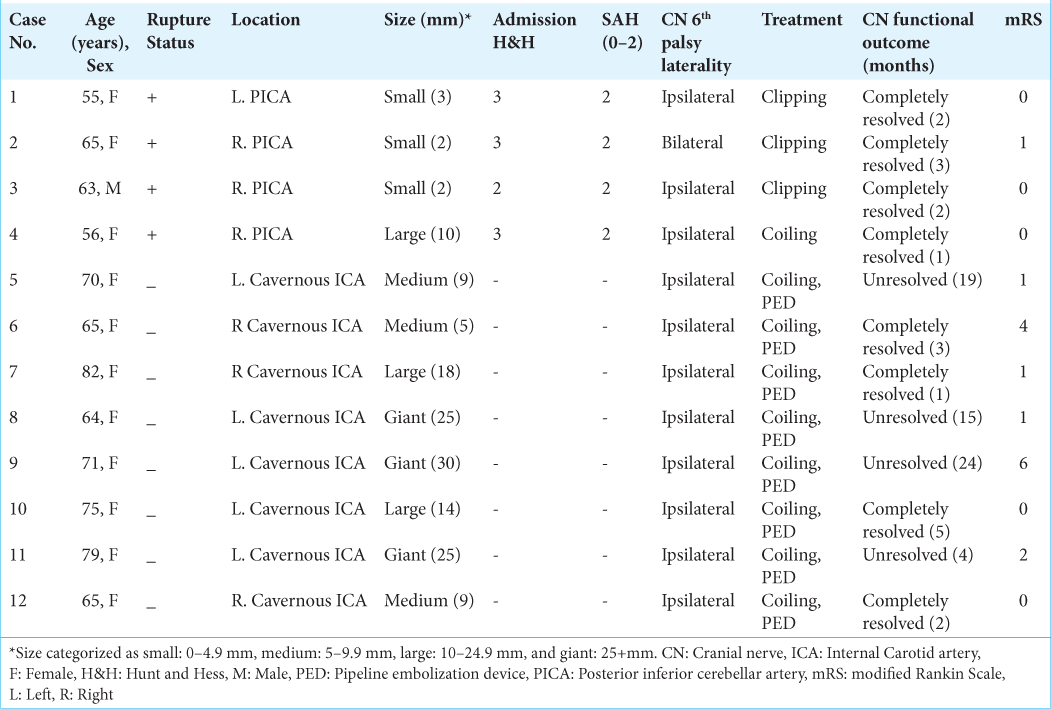
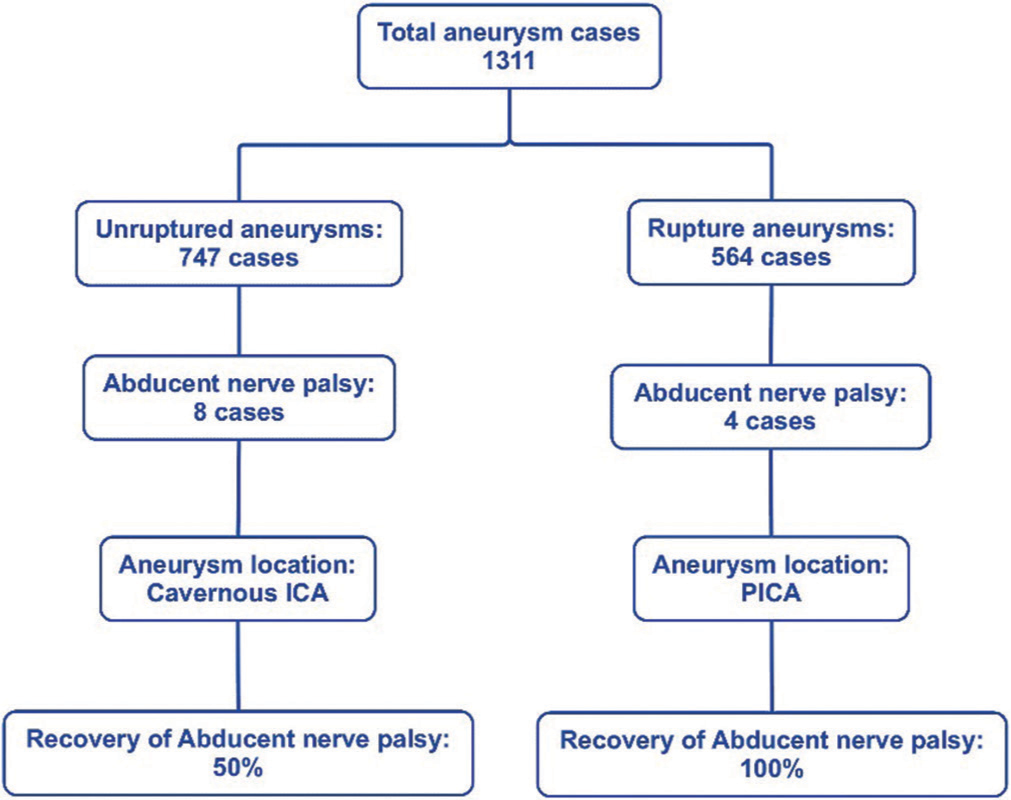
Results: Out of 1311 cases analyzed, a total of 12 cases were identified as having CN 6th palsy at the presentation. Eight out of the 12 were found in the unruptured aneurysm in the cavernous segment of the internal carotid artery (ICA). The other four cases of CN 6th palsy were found in association with ruptured aneurysms located exclusively at the posterior inferior cerebellar artery (PICA). For the full functional recovery of the CN 6th palsy, there was 50% documented full recovery in the eight cases of the unruptured cavernous ICA aneurysm. On the other hand, all four patients with ruptured PICA aneurysms have a full recovery of CN 6th palsy. The duration for recovery for CN palsy ranges from 1 to 5 months.
Conclusion: The association between intracranial aneurysms and CN 6th palsy at presentation may suggest distinct patterns related to aneurysmal location and size. The abducent nerve palsy can be linked to unruptured cavernous ICA and ruptured PICA aneurysms. The recovery of CN 6th palsy may be influenced by aneurysm size, rupture status, location, and treatment modality.
Keywords: Abducent nerve, Cranial nerve palsy, Intracranial aneurysms
INTRODUCTION
Cranial nerve (CN) palsy can be observed in patients with intracranial aneurysms as an initial disease manifestation or a result of the treatment. As a presenting finding, CN palsy can be a component of the constellation of symptoms encountered in patients with cerebral aneurysms.[
The anatomical proximity forms the background for the association between a CN palsy and an aneurysm. Classic examples include oculomotor nerve palsy associated with a posterior communicating artery aneurysm.[
The abducent nerve follows a peculiar intracranial pathway and is susceptible to being affected by an enlarged aneurysm along the distance from the brainstem to the orbit. There are potentially vulnerable compression/entrapment points for the abducent near the petrous apex posteriorly and in the lateral wall of the cavernous sinus anteriorly. Thus, CN 6th palsy can be linked to aneurysms originating from arteries with close anatomical proximity.[
MATERIALS AND METHODS
A retrospective chart review was conducted at the University of Pittsburgh Medical Center from June 2016 to July 2023, with an emphasis on CN 6th palsy at presentation while excluding cases of postprocedural CN 6th palsy. The study only included newly diagnosed aneurysms ruptured or unruptured aneurysms undergoing initial treatment; previously treated/retreated aneurysms were excluded from the study. Patients with mycotic aneurysms were also excluded from the study. The study period, inclusion, and exclusion criteria were defined as per this objective. The following variables were extracted and analyzed: demographics (age and sex), clinical characteristics (ruptured status, HTN, diabetes mellitus [DM], and CN 6th palsy), aneurysm characteristics (location, size categorized as small: 0–4.9 mm, medium: 5–9.9 mm, large: 10–24.9 mm, and giant: 25+ mm), for ruptured aneurysms (Hunt and Hess score categorized as score 1: minimal symptoms, score 2: moderate-to-severe headaches, score 3: confusion or mild neurological issues, score 4: stupor and possible weakness, and score 5: deep coma, and subarachnoid hemorrhage [SAH] severity score characterized as score 0: no blood, score 1: small amount of blood, score 2: moderate amount of blood, score 3: completely filled with blood) treatment modalities (clipping, coiling, pipeline embolization device [PED], and other treatments as applicable), and outcome variables for CN resolution status, follow-up duration of the CN 6th palsy, and functional outcome of the patient modified Rankin scale (mRS) categorized as a good outcome for the scores of 0–2, while a poor outcome for 3–6 scores. Data were collected from electronic health records, and the variables were included in the study. Any discrepancies in the data were resolved through a thorough review of the medical records. Retrospective data collection was approved by the University of Pittsburgh’s Institutional Review Board. All patients underwent standard surgical consent.
Descriptive statistics were first employed to summarize the continuous and categorical variables, including frequencies and percentages for sex, DM, ruptured status, CN 6th palsy, location of the aneurysm, size, and treatment modalities, as well as the mean and standard deviation (SD) for age. Subsequently, CN 6th palsy and aneurysm location are associated with categorical variables such as sex, ruptured status, DM, mRS, and aneurysm Size were explored using Chi-square tests. Finally, to investigate the factors associated with CN 6th palsy in aneurysm patients, univariate logistic regression analyses were performed. The predictors analyzed were sex, ruptured status, DM, aneurysm location, size, and treatment. P < 0.05 is considered significant. IBM Statistical Package for the Social Sciences Statistics version 25 software was used in the analysis.
RESULTS
In this study, 1311 patients with previously untreated intracranial aneurysms who were treated at the University of Pittsburgh Medical Center from June 2016 to July 2023 were included in the study. The general patient characteristics were as follows: 564 cases accounted for ruptured (43.0%), and 747 cases for unruptured aneurysms (57.0%). The mean age was 58.6 years (SD = 13.1), with 71.6% females. The most frequently identified aneurysm location was the anterior communicating artery (Acom) at 23.0%. The cavernous internal carotid artery (ICA) represented 1.7% (22 cases), and the posterior inferior cerebellar artery (PICA) represented 5.1% (67 cases). About 46.1% were treated with microsurgical clipping, and 53.9% had neuroendovascular treatment.
In our study, CN 6th palsy was observed in 0.9% (12 cases) of the total 1311 cases. Further, analysis of the abducent nerve revealed that in the unruptured category, all the aneurysms were located in the cavernous ICA. Among the 22 cases of treated cavernous ICA aneurysms, 8 individuals (36.4%) presented with CN 6th palsy, while 14 patients (63.6%) did not. Aneurysm size displayed differences, with the CN 6th palsy group having a higher proportion of giant-sized aneurysms in 3 cases (13.6%) compared to the CN 6th non-palsy group in 2 cases (9.1%). Conversely, the CN 6th non-palsy group had a higher number of large-sized aneurysms, with 8 cases (36.4%), compared to the CN 6th palsy group with 2 cases (9.1%). Furthermore, in the analysis of this group, the aneurysm size showed significant associations with CN 6th palsy (P < 0.001) [
The analysis of abducent nerve palsy at presentation revealed that in the ruptured category, all the aneurysms were PICA (four cases). Those four cases represent 6.0% of total PICA (67 cases) and 6.8% of ruptured PICA patients. For aneurysm size, in the CN 6th palsy group, small-sized aneurysms were found in three cases, and one patient had a large-sized aneurysm. In our statistical analysis of the PICA aneurysm group, no statistically significant associations were found regarding the factors in the study [
Logistic regression confirmed that the presence of a cavernous ICA aneurysm is associated with an increased likelihood of abducent palsy (odd ratio [OR] = 183.4, P <0.001), as did PICA aneurysm (OR = 9.8, P < 0.001). In addition, aneurysmal size (OR = 2.8, P = 0.005) significantly correlated with abducent nerve palsy. However, factors such as sex (OR = 4.3, P = 0.157), rupture status (OR = 0.6, P = 0.499), and the presence of DM (OR = 0.000, P = 0.996) did not yield statistically significant correlations with CN 6th palsy [
For treatment strategies and outcomes, in the ruptured/PICA cases, three out of four patients underwent clipping, while one patient underwent coiling. Resolution of the CN 6th palsy was seen within 1–3 months in all ruptured cases with a 2-month average recovery time. All had good functional outcomes (mRS 0–2) at follow-up. In unruptured/cavernous ICA cases, all eight patients underwent coiling and PED placement. A median follow-up period was 15.5 months, ranging from 4 to 24 months. The mRS of this group revealed that six patients had good outcomes, and two had poor ones. This cavernous ICA group has a 50.0% resolution rate (four cases) with a 2.7-month average recovery time and an equal number of unresolved instances (four cases) of CN 6th palsy.
In resolved cases, all associated aneurysms were classified as medium or large, with two cases falling into each category. Conversely, among the unresolved cases, three of these cases were with giant-sized aneurysms, while only one showed an aneurysm of medium size. For aneurysms, a similar distribution of outcomes was observed in unresolved cases [
DISCUSSION
The abducent nerve emerges from the pontomedullary junction and proceeds to enter the prepontine cistern. It subsequently follows a relatively vertical course along the dorsal surface of the clivus adjacent to the petrous apex. Continuing its course, the nerve gains access to the cavernous sinus through Dorello’s canal, eventually entering the orbit through the superior orbital fissure.[
According to the findings of our study, a noteworthy association has been established between the abducent nerve and ruptured PICA aneurysms. While, in the context of unruptured aneurysms, a statistically significant correlation has been identified between the abducent nerve palsy and factors such as the cavernous location, and dome size. An anatomical understanding of the aneurysm locations in our study that presented with 6th CN palsy is described in [
Correlation between abducent nerve palsy and unruptured intracranial aneurysms
In our study, eight cases with abducent nerve palsy were found to be associated with unruptured cavernous ICA aneurysms with 75% related to giant dome size. In a comparable study conducted by Durner et al., a total of 36 unruptured giant cavernous ICA aneurysms were examined within a cohort of 34 patients, 25 individuals had CN dysfunction. The findings indicated that abducent nerve palsy manifested in 84% of the patients. Among these cases, 6th nerve palsy exclusively presented in 44% of the patients as the sole CN impairment.[
The potential mechanisms by which an aneurysm can result in dysfunction of the abducent nerve encompass direct compression due to the nerve’s proximity to the ICA within the cavernous sinus. In addition, occlusion of the feeding vessels supplying the structures of the cavernous sinus may occur due to either compression by the aneurysm or thrombosis and may cause an acute simultaneous ischemic lesion of the CNs.[
Correlation between abducent nerve palsy and ruptured intracranial aneurysms
Four cases in the ruptured aneurysm category were found to have CN 6th palsy at presentation, with all located at the PICA junction with vertebral artery (VA). This aligns with the work of Burkhardt et al., who investigated 13 patients with 6th nerve palsy from a broader cohort of 106 patients displaying PICA aneurysms.[
Due to the close anatomical proximity of the abducent nerve’s origin at the pontomedullary junction to that of the PICA from the VA, a PICA aneurysm holds the potential to serve as a compressive element affecting the 6th CN at the root exit zone.[
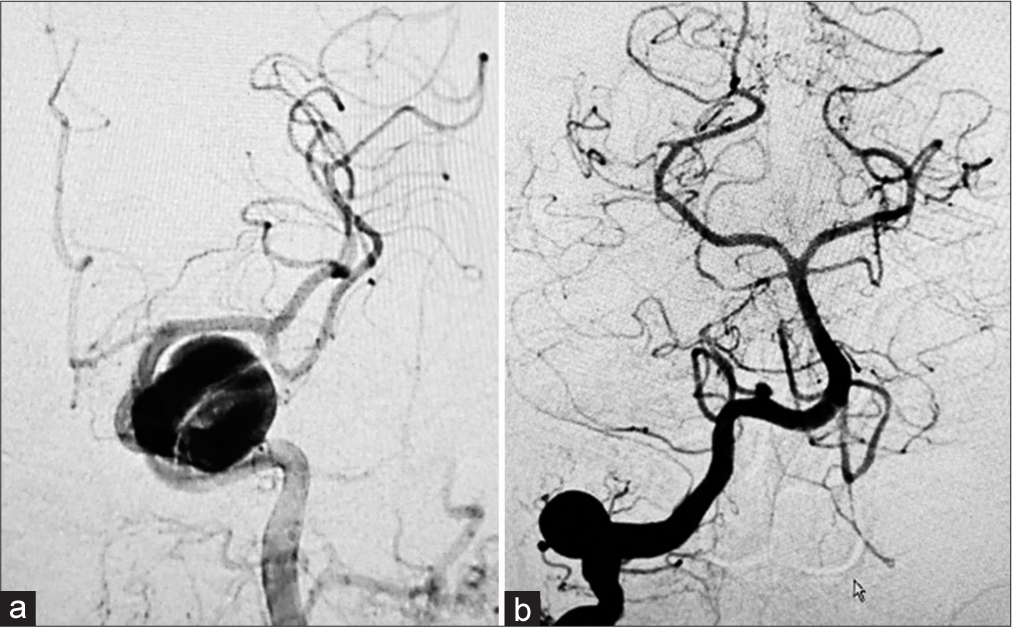
Figure 3:
Illustrative depiction of the abducent nerve relation to various aneurysms: (a) cavernous ICA aneurysm (white asterisk) and (b) PICA aneurysm (black asterisk). BA: Basilar artery, VI: Abducent nerve, PICA: Posterior inferior cerebellar artery, ICA: Internal carotid artery, CS: Cavernous sinus. Illustration prepared by Ahmed Muthana and courtesy of Samer Hoz.
Laterality of abducent nerve palsy associated with intracranial aneurysms
The distinction between bilateral and unilateral occurrences of abducent palsy hinges on a multitude of factors. Unilateral palsy primarily arises due to direct compression of the aneurysm on the neuronal fibers, with the resulting condition being either ipsilateral or contralateral to the affected side and this can be applied regardless of the rupture status of the aneurysm.[
Functional recovery of abducent nerve palsy in the setting of intracranial aneurysms
CNs typically require a certain duration to regain their functionality following the treatment of the underlying cause. Surgical or endovascular interventions targeted at treating caused aneurysm, offer the potential for recovery of nerve function. Nonetheless, the scope and speed of recuperation can exhibit significant variability among patients. The literature showed quite a variability in the period of regaining function of the CN palsy in the setting of intracranial aneurysms. This is because the factors contributing to the nature of recovery encompass the degree of nerve compression, the duration of symptoms before treatment, and the efficacy of the intervention in alleviating aneurysm-induced pressure on the nerve.[
The general anticipation of the functional recovery in aneurysm-induced-CN palsy is controversial with the actual impact of treatment modality on the CN palsy outcome is difficult to be precisely determined using a small sample size. While some patients may encounter full or nearly complete restoration of function, others could be left with residual deficits.[
The variation observed in CN recovery between ruptured and unruptured cases could be attributed to prolonged compression by the aneurysm within confined spaces, rendering the CN less prone to recovery. This notion is substantiated by the size of the aneurysms in unresolved cases, with 75% of them being classified as giant aneurysms. As previously elucidated, the plausible mechanism underlying this phenomenon involves the occlusion of feeding vessels supplying the abducent nerve, thereby inducing acute ischemic lesions within the nerve simultaneously. Furthermore, the type of treatment can potentially influence the functional regaining of CN palsy.
Treatment options may directly impact the recovery of 6th nerve palsy; it is noteworthy that surgical clipping offers prompt relief from compression, regardless of the specific surgical technique employed, whether involving clipping alone or in conjunction with aneurysm sac decompression. Endovascular coiling may not alleviate the entirety of the mass effect, some studies mentioned that the non-compressible coil mass might potentially contribute to an increase in pressure. However, recent studies mentioned that the mass effect remains after endovascular packing, and CN palsies improve comparably to the recovery observed after clipping.[
A study focused on the long-term functional outcomes of CN palsy underscores the observation that endovascular treatment of cavernous ICA aneurysms tends to render the abducent palsy less likely to resolve.[
In managing persistent symptoms after a significant duration, usually more than 3 months, and ameliorating functional outcomes, therapeutic approaches such as eye patching, prismatic glasses or botulinum toxin injections can be employed.[
Based on the observations, CN 6th palsy at the time of presentation is linked to cavernous ICA aneurysms in unruptured cases, while in ruptured cases, it is associated with PICA aneurysms. A discernible pattern emerges, indicating a greater likelihood of CN function recovery in ruptured aneurysm cases than in unruptured ones.
While this study effectively explores the link between CN palsy and aneurysms, the multifactorial etiology of CN palsy could not be exhaustively addressed. This condition often arises from a complex interplay of anatomical, physiological, and potentially genetic factors, extending beyond aneurysm presence alone.
The relationship between CN 6th palsy and treatment or mRS cannot be directly established, as these CNs were assessed at the time of presentation and could not be tested within the scope of our study regarding treatment and outcomes. This applies even if there is statistical significance; it does not necessarily imply strong correlations. Instead, it serves as an indicator of a correlation, irrespective of its level of significance.
Limitations
This study possesses certain limitations that warrant consideration when interpreting the results. The sample size comprised 12 patients with CN 6th palsy, in the form of four patients out of 67 with PICA and eight patients out of 22 with cavernous ICA aneurysms, which, while substantial, might not fully represent the diverse spectrum of patient and aneurysm characteristics present in the broader population. Consequently, the generalizability of the findings to larger and more varied patient groups could be limited.
CONCLUSION
There is a significant association between the abducent nerve and intracranial aneurysms, illuminating distinct patterns concerning the location and size of the aneurysm. A robust connection has been observed between the abducent nerve and ruptured PICA aneurysms. In addition, within the realm of unruptured aneurysms, there are compelling correlations, particularly about the cavernous location and size of the aneurysm.
Disclosures
The data and analytic code used in this study are available from the corresponding authors upon reasonable request.
Ethical approval
The research/study was approved by the Institutional Review Board at UPMC, number 2210096, dated 2023.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
Dr. Gross is a consultant for Medtronic, Stryker, and MicroVention.
Dr. Nogueira reports consulting fees for advisory roles with Stryker Neurovascular, Cerenovus, Medtronic, Phenox, Anaconda, Genentech, Biogen, Prolong Pharmaceuticals, Imperative Care, and stock options for advisory roles with Brainomix, Viz-AI, Corindus Vascular Robotics, Vesalio, Ceretrieve, Astrocyte, and Cerebrotech.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
Acknowledgments
We acknowledge Ahmed Muthana for providing the graphical illustration.
References
1. Burkhardt JK, Winkler EA, Lasker GF, Yue JK, Lawton MT. Isolated abducent nerve palsy associated with subarachnoid hemorrhage: A localizing sign of ruptured posterior inferior cerebellar artery aneurysms. J Neurosurg. 2017. 128: 1830-8
2. Dimopoulos VG, Fountas KN, Feltes CH, Robinson JS, Grigorian AA. Literature review regarding the methodology of assessing third nerve paresis associated with non-ruptured posterior communicating artery aneurysms. Neurosurg Rev. 2005. 28: 256-60
3. Durner G, Piano M, Lenga P, Mielke D, Hohaus C, Guhl S. Cranial nerve deficits in giant cavernous carotid aneurysms and their relation to aneurysm morphology and location. Acta Neurochir. 2018. 160: 1653-60
4. Elder C, Hainline C, Galetta SL, Balcer LJ, Rucker JC. Isolated abducent nerve palsy: Update on evaluation and diagnosis. Curr Neurol Neurosci Rep. 2016. 16: 69
5. Garg K, Singh PK, Mahapatra AK, Sharma BS. Bilateral abducent nerve palsy associated with subarachnoid hemorrhage. Br J Neurosurg. 2014. 28: 771-5
6. Geçirilmesi G. Review of a series with abducent nerve palsy. Turk Neurosurg. 2008. 18: 366-73
7. Hyland HH, Barnett HJ. The pathogenesis of cranial nerve palsies associated with intracranial aneurysms. Trans Am Neurol Assoc. 1953. 3: 127-30 discussion, 130-1
8. Jeon JS, Lee SH, Son YJ, Chung YS. Slowly recovering isolated bilateral abducent nerve palsy after embolization of ruptured anterior communicating artery aneurysm. J Korean Neurosurg Soc. 2013. 53: 112-4
9. Jha RM, Klein JP. Clinical anatomy and imaging of the cranial nerves and skull base. Semin Neurol. 2012. 32: 332-46
10. Koskela E, Laakso A, Kivisaari R, Setälä K, Hijazy F, Hernesniemi J. Eye movement abnormalities after a ruptured intracranial aneurysm. World Neurosurg. 2015. 83: 362-7
11. Kunz M, Dorn F, Greve T, Stoecklein V, Tonn JC, Brückmann H. Long-term functional outcome of symptomatic unruptured intracranial aneurysms in an interdisciplinary treatment concept. World Neurosurg. 2017. 105: 849-56
12. Laun A, Tonn JC. Cranial nerve lesions following subarachnoid hemorrhage and aneurysm of the circle of Willis. Neurosurg Rev. 1988. 11: 137-41
13. Markwalder TM, Meienberg O. Acute painful cavernous sinus syndrome in unruptured intracavernous aneurysms of the internal carotid artery: Possible pathogenetic mechanisms. J Neuroophthalmol. 1983. 3: 31-6
14. Nishino K, Ito Y, Hasegawa H, Shimbo J, Kikuchi B, Fujii Y. Development of cranial nerve palsy shortly after endosaccular embolization for asymptomatic cerebral aneurysm: Report of two cases and literature review. Acta Neurochir (Wien). 2009. 151: 379-83
15. Rush JA, Younge BR. Paralysis of cranial nerves III, IV, and VI: Cause and prognosis in 1,000 cases. Arch Ophthalmol. 1981. 99: 76-9
16. Smith DE, Blasi A. Acquired abducens nerve palsy secondary to tuberculosis. Optometry. 2009. 80: 567-71
17. Tawk RG, Villalobos HJ, Levy EI, Hopkins LN. Surgical decompression and coil removal for the recovery of vision after coiling and proximal occlusion of a clinoidal segment aneurysm: A technical case report. Neurosurgery. 2006. 58: E1217 discussion E17
18. Wen A, Cao WF, Liu SM, Zhou YL, Xiang ZB, Hu F. Incidence and risk factors of cranial nerve palsy in patients with tuberculous meningitis: A retrospective evaluation. Infect Drug Resist. 2023. 16: 829-41