- Department of Neurosurgery, Jacobs School of Medicine and Biomedical Sciences, University at Buffalo, State University of New York, New York, USA
- Department of Neurosurgery, Buffalo General Medical Center at Kaleida Health Buffalo, New York, USA
Correspondence Address:
Douglas B. Moreland
Department of Neurosurgery, Jacobs School of Medicine and Biomedical Sciences, University at Buffalo, State University of New York, New York, USA
Department of Neurosurgery, Buffalo General Medical Center at Kaleida Health Buffalo, New York, USA
DOI:10.4103/sni.sni_133_17
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: John F. Morrison, Kunal Vakharia, Douglas B. Moreland. Lumbar laminectomy in a captive, adult polar bear (Ursus maritimus). 13-Jun-2017;8:112
How to cite this URL: John F. Morrison, Kunal Vakharia, Douglas B. Moreland. Lumbar laminectomy in a captive, adult polar bear (Ursus maritimus). 13-Jun-2017;8:112. Available from: http://surgicalneurologyint.com/surgicalint-articles/lumbar-laminectomy-in-a-captive-adult-polar-bear-ursus-maritimus/
Abstract
Background:Animals held in captivity tend to live longer than do their wild counterparts, and as such, are prone to developing age-related degenerative injuries. Here, we present a case of an adult female polar bear with symptomatic lumbar stenosis. There is a paucity of literature on large mammalian spine surgery, and anatomical differences between humans and other vertebrates must be taken into consideration.
Case Description:A 24-year-old female polar bear residing at the zoo was found to have decreased motor function in her hind legs. Diagnostic myelography performed at the L7/S1 level demonstrated lumbar stenosis at L5/6 for which a laminectomy was performed. Postoperatively, she returned to premorbid functional level, with no apparent associated adverse sequelae.
Conclusions:To our knowledge, this is the first reported case of spine surgery in a polar bear and demonstrates that neurosurgical diagnostic and operative techniques developed for humans can also be applied to large mammals with successful results.
Keywords: Large animal neurosurgery, lumbar laminectomy, polar bear spine surgery
INTRODUCTION
Indigenous to the Arctic Circle, the polar bear, or Ursus maritimus, is a common feature of zoological parks throughout the world. Life expectancy in the wild ranges into the second decade.[
Spinal spondylosis and subsequent stenosis result in a significant number of emergency department visits and operative procedures in humans. Symptoms are often subtle, chronic, and progressive due to the degenerative, age-, and use-related nature of the disease. Depending on the location of the lesion, symptoms can include radiculopathy or myelopathy. Spondylosis tends to be a more common pathologic condition among humans because of our erect posture, lack of natural predators, and long-life expectancy, which makes diagnosis slightly more difficult and rare in quadrupeds such as bears.
Although all mammalian species possess 7 cervical vertebrae, many anatomical variations among mammalian species have been noted. For example, the polar bear has 13 thoracic vertebrae and 7 lumbar vertebrae. Further, although the spinal cord in adult humans descends to approximately the L1 level, in many vertebrate animals, the terminal portion of the spinal cord is at L6 or L7.[
CASE REPORT
A captive, 450 kg, 24-year-old female polar bear, “Becky,” was noted to be listless, have decreased ambulation, and experiencing particular difficulty with hind leg function [
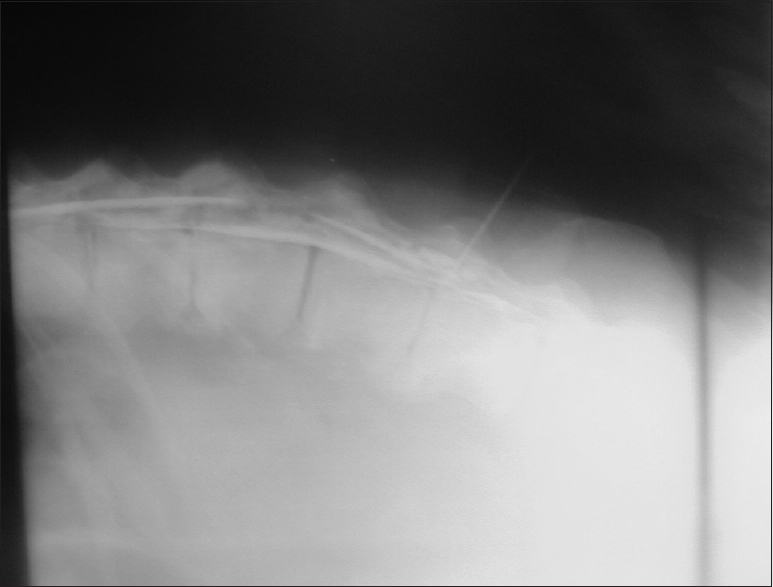
Radiological findings
Ketamine anesthesia was administered via pneumatic tranquilizer dart to the subcutaneous or intramuscular space. Dosing was adjusted to sedation response after an interval of 10–15 min. Becky was then transported to the zoo infirmary for myelography. A spinal injection of 80 ml of Isovue contrast material (Bracco Diagnostic Inc., Milan, Italy) via an 18-gauge, 6.5-inch discogram needle at the L7–S1 interspinous level demonstrated significant spinal stenosis at L5/6 on lateral X-ray [
Surgical technique
Fur at the surgical site was clipped and the wound was prepared and draped in usual sterile fashion [
Postoperative course
Becky was returned to her environment and emerged from anesthesia successfully. Over the course of the next week, she returned to ambulation with apparent full strength and started to eat, behave more normally, and gain weight [
DISCUSSION
Literature on comparative anatomy of the bear spine and associated disorders is sparse. In the black bear, there have been reports of spinal decompression as well as magnetic resonance imaging examination and histopathology evaluation in an animal with thoracic disc herniation that was ultimately sacrificed.[
Much of the literature on management of compressive spinal disorders in quadrupeds is based on experience in canines. Conservative therapy, consisting of confinement and restriction of movement, is more difficult in larger, more aggressive animals. It is equally difficult to make a diagnosis of spinal stenosis in horizontally erect animals. Surgical intervention and postoperative rehabilitation are also more complicated due to larger animals having more tissue, which increases the depth of the field for surgical decompression.[
Anesthesia and surgical care in large, predatory animals can pose unique challenges. The goal of the anesthetic agent, immobilization and analgesia, and in large animals, this can be problematic. The dosing is both weight-based and absorption variable between different tissue types accessed (skin, fat, muscle, vessels); too small dose results in lighter sedation (animal movement during the procedure and risk to both the animal and surgeon) whereas too large risks respiratory compromise.
Diskectomy and laminectomy and the effects of these procedures on spinal stability in quadruped animals have not been investigated. In this case, we applied our knowledge of human and canine anatomy. Preservation of the disk space with simple posterior decompression was performed. The ligamentous structures around the vertebrae (anterior and posterior longitudinal ligaments and facet joint capsules) were kept intact whereas those in the central canal were removed for the decompression, as in simple laminectomies in humans. Polar bear and canine anatomy and neural connectivity were considered similar, thus, a simple laminectomy with resection of ligamentous hypertrophy was chosen because of the posterior compression of the cord. The true biomechanical cause of this injury is still poorly understood because fewer studies have evaluated stresses and strains on the spine and facets in bears than they have in humans.
CONCLUSION
Technical progress of surgical intervention for thoracolumbar stenosis in humans has resulted in minimally invasive approaches and decreased procedure time, as well as shortened hospital stays or same-day surgical care. Given their expertise in spinal and cranial surgery, neurosurgeons are well-equipped to support veterinary colleagues in dealing with new and complex issues that are rarely seen in these animals. The authors feel that it is our privilege to use our knowledge and abilities for both mankind and large mammals. Spine surgery has been reported in several other animal species, including dog, cat, gorilla, lion, and black bear.[
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors thank Allen W. Prowten DVM for providing veterinary care, Paul H. Dressel BFA for preparation of the illustrations, and Carrie Owens MSILS for editorial assistance.
References
1. Amstrup S, Feldhamer GT, Chapman BJ. The Polar Bear - Ursus maritimus. Wild Animals of North America. 2003. p.
2. Aryan HE, Jandial R, Nakaji P, Greenberg MS, Janssen DL, Huang J. Lumbar diskectomy in a human-habituated mountain gorilla (Gorilla beringei beringei). Clin Neurol Neurosurg. 2006. 108: 205-10
3. Wrigley RE. The Oldest Living Polar Bear. Polar Bears Int. 2008. 15: 4-
4. Galloway DS, Coke RL, Rochat MC, Radinsky MAG, Hoover JP, Carpenter JW. Spinal compression due to atlantal vertebral malformation in two African lions (Panthera leo). J Zoo Wildl Med. 2002. 33: 249-55
5. Knafo SE, Divers SJ, Rech R, Platt SR. Magnetic resonance imaging diagnosis of intervertebral disc disease and myelomalacia in an American black bear (Ursus americanus). J Zoo Wildl Med. 2012. 43: 397-401
6. Nichols JB, Dulisch ML, Sikarskie JG, McNamara MA. Spinal decompression in a black bear. J Am Vet Med Assoc. 1980. 177: 882-4
7. Ramirez O, Thrall DE. A review of imaging techniques for canine cauda equina syndrome. Vet Radiol Ultrasound. 1998. 39: 283-96