- Department of Surgery, Division of Neurosurgery, University Malaya Medical Centre, Petaling Jaya, Malaysia
- Department of Neurosurgery, Gleneagles Hospital Kuala Lumpur, Kuala Lumpur, Malaysia
- Department of Neurosurgery, Hospital Kuala Lumpur, Kuala Lumpur, Malaysia.
Correspondence Address:
Hari Chandra Thambinayagam, Department of Surgery, Division of Neurosurgery, University Malaya Medical Centre, Petaling Jaya, Malaysia.
DOI:10.25259/SNI_366_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Bih Huei Tan1, Regunath Kandasamy2, Siti Azleen Mohamad3, Hari Chandra Thambinayagam1. Predictors of radiation-induced changes in arteriovenous malformation patients undergoing radiosurgery: Insights from a Malaysian linear accelerator cohort. 28-Jun-2024;15:223
How to cite this URL: Bih Huei Tan1, Regunath Kandasamy2, Siti Azleen Mohamad3, Hari Chandra Thambinayagam1. Predictors of radiation-induced changes in arteriovenous malformation patients undergoing radiosurgery: Insights from a Malaysian linear accelerator cohort. 28-Jun-2024;15:223. Available from: https://surgicalneurologyint.com/surgicalint-articles/12964/
Abstract
Background: Radiation-induced changes (RICs) post-stereotactic radiosurgery (SRS) critically influence outcomes in arteriovenous malformation (AVM) treatments. This study aimed to identify predictors of RICs, described the types and severity of RICs, and assessed their impact on patient’s functional outcomes to enhance risk assessment and treatment planning for AVM patients.
Methods: This retrospective study analyzed 87 AVM patients who underwent SRS at Hospital Kuala Lumpur between January 2015 and December 2020. RICs were identified through detailed magnetic resonance imaging evaluations, and predictive factors were determined using multiple logistic regression. Functional outcomes were assessed with the modified Rankin scale (mRS).
Results: Among the cohort, 40.2% developed RICs, with radiological RICs in 33.3%, symptomatic RICs in 5.7%, and permanent RICs in 1.1%. Severity categorization revealed 25.3% as Grade I, 13.8% as Grade II, and 1.1% as Grade III. Notably, higher Pollock–Flickinger scores and eloquence location were significant predictors of RIC occurrence. There was a significant improvement in functional outcomes post-SRS, with a marked decrease in non-favorable mRS scores from 8.0% pre-SRS to 1.1% post-SRS (P = 0.031).
Conclusion: The study identified the eloquence location and Pollock–Flickinger scores as predictors of RICs post-SRS. The significant reduction in non-favorable mRS scores post-SRS underscores the efficacy of SRS in improving patient outcomes. Their results highlighted the importance of personalized treatment planning, focusing on precise strategies to optimize patient outcomes in AVM management, reducing adverse effects while improving functional outcomes.
Keywords: Arteriovenous malformation, Linear accelerator, Radiation-induced changes, Stereotactic radiosurgery
INTRODUCTION
Cerebral arteriovenous malformations (AVMs) are abnormalities of the intracranial vessels, which consist of a number of direct connections between the arterial and venous systems without an intervening capillary bed.[
Stereotactic radiosurgery (SRS) has been shown to be an excellent treatment modality for patients with small-to-moderated AVM. Complete angiographic obliteration can be achieved in 80–90% of cases with a latency period of 2–3 years, which has been considered effective as surgical resection.[
The earliest and most frequently observed complication after SRS for AVMs is radiation-induced changes (RICs), which typically develop 6–18 months after radiosurgery. [
Our study aimed to determine the incidence and predictors of RICs in post-SRS AVM patients. In addition, our secondary objectives were to describe the demographic and clinical characteristics of post-SRS AVM patients, describe the type of RICs developed post-SRS, and determine the effect of RICs on post-SRS AVM patients based on functional outcomes using a modified Rankin scale (mRS). The comprehensive insights gained from this study are intended to improve patient management and outcomes by refining treatment approaches for AVM patients undergoing SRS, thereby enhancing overall treatment efficacy.
MATERIALS AND METHODS
Patient selection
This is a retrospective study that obtained approval from the Local Institutional Review Board. We identified patients diagnosed with AVM and treated with SRS alone or in combination with another modality from clinic records. The study included all adult AVM patients treated with SRS at Hospital Kuala Lumpur (HKL) between January 1, 2015, and December 31, 2020. Patients aged over 18 years with AVM confirmed by digital subtraction angiography (DSA) and/or magnetic resonance imaging (MRI)/magnetic resonance-angiogram (MRA)/magnetic resonance-venogram were included in the study. Patients must have received treatment and/or follow-up at the Neurosurgery Department of HKL, with a follow-up period of at least 2 years after SRS and undergone SRS alone or in combination with another treatment modality. Exclusion criteria, including patients lost to follow-up post-SRS and those who were clinically followed but lacked available MRI imaging, including T2 and T2 fluid-attenuated inversion recovery (FLAIR) sequences for analysis, were excluded from the study.
Variables
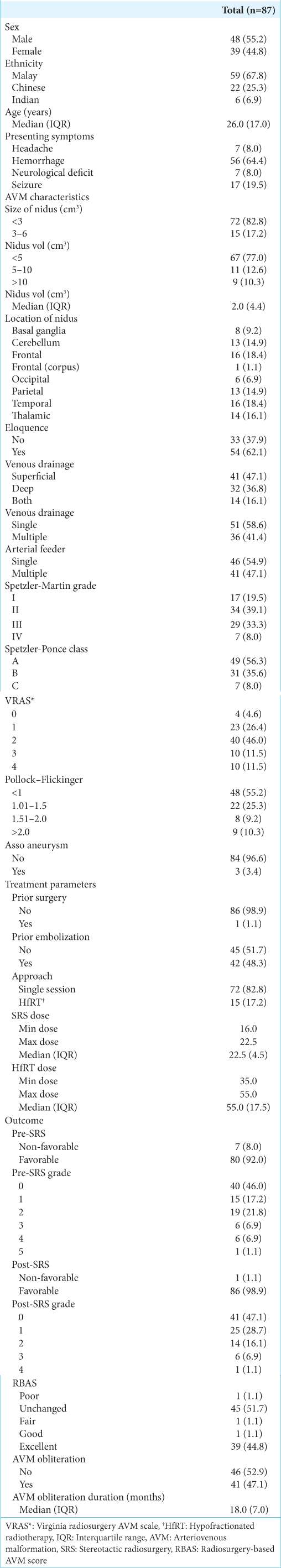
We evaluated patient demographics (age, gender, and race), clinical presentation, AVM characteristics (size of nidus, volume of nidus, nidus location, eloquence, venous drainage, arterial feeder, Spetzler-Martin (SM) grading, Spetzler-Ponce Class, and presence of associated aneurysm), treatment parameters (prior surgery, prior embolization, approach, and treatment dose), along with clinical, angiographic, and MRI follow-up periods.
Radiosurgical technique
A multidisciplinary meeting was convened to discuss definitive treatment options for patients. This team comprised neurosurgeons, oncologists, and interventional radiologists. Each team member reviewed the patient’s records, and a consensus was reached regarding the definitive treatment plan. Linear accelerator radiosurgery was utilized at HKL and the National Cancer Institute (Institute Kanser Negara, IKN) with a frameless-based face mask. Stereotactic cerebral angiography, integrating MRA and DSA, was incorporated into the treatment planning process. Neurosurgeons conducted nidus definition and contouring, while radiation oncologists performed dose planning based on AVM characteristics, proximity to critical structures, and history of prior radiation therapy.
Follow-up
Following SRS, patients were discharged and subsequently followed up in the clinic to assess clinical symptoms and review MRI imaging. Any newly reported symptoms deemed to be related to radiosurgery by the treating physician were categorized as radiation-induced neurologic signs and symptoms. MRI, including contrast MRI, T2, and T2 FLAIR sequences, as well as MRA, were conducted post-radiosurgery. Once the MRI confirmed total obliteration of the AVM nidus, DSA was performed. Consistent with our hospital protocol, which aligned with existing literature, RICs typically manifest within 6–18 months after radiosurgery, mirroring our MRI follow-up schedule at 6-month intervals until obliteration, followed by annual evaluations after that. [
Outcomes
The primary endpoints of this study were as follows: (1) to assess the incidence of RICs, (2) to quantify the type and severity of RICs, and (3) to identify predictors associated with the development of RICs. Secondary endpoints included describing the demographic and clinical characteristics of post-SRS AVM patients and evaluating the impact of RICs on patients’ outcomes.
RICs, as defined by Yen et al., were newly developed areas of hyperintensity in the T2 signal surrounding the treated AVM nidi following radiosurgery.[
The secondary endpoint focused on the functional status of patients at 24 months post-SRS. Pre-SRS and post-SRS mRS scores were utilized for their simplicity, widespread acceptance, and established reliability and validity.[
The radiosurgery-based grading system categorized patient outcomes as excellent, good, fair, unchanged, or poor. Excellent outcomes signified complete nidus obliteration without new deficits, whereas good outcomes indicated minor deficits not significantly impacting daily activities. Fair outcomes reflected major deficits leading to a decline in functioning despite AVM obliteration. Unchanged outcomes indicated persistent arteriovenous shunting without new deficits, whereas poor outcomes encompassed new deficits and incomplete nidus obliteration.[
Statistical analysis
Categorical variables were presented as frequencies and percentages, while the normality of continuous variables was assessed using the Shapiro–Wilk test. Skewed distribution variables were described using the median and interquartile range, whereas normally distributed variables were expressed as mean and standard deviation. The association between demographic factors, AVM characteristics, treatment parameters, and outcomes in RIC and non-RIC groups was evaluated using the Chi-square test, with Fisher’s Exact test applied when assumptions for the Chi-square test were not met. Medians between RIC and non-RIC groups were compared using the Mann–Whitney Rank U-test. Multiple logistic regression was employed to identify predictors of RIC, considering variables with P-value of at least 0.250 from binary logistic regression for multivariate analysis. The multivariate analysis included factors such as nidus location, depth (deep/superficial), venous drainage pattern (single/multiple), SM grade, Spetzler-Ponce class, radiosurgery-based AVM score (RBAS), approach, AVM obliteration, and AVM duration. Changes in mRS, grade, and RBAS pre- and post-SRS were assessed using the McNemar test. Statistical analyses were conducted using the Statistical Package for the Social Sciences software (version 26), with P ≤ 0.05 considered statistically significant. Interrater bias was calculated using Cohen’s Kappa value.
RESULTS
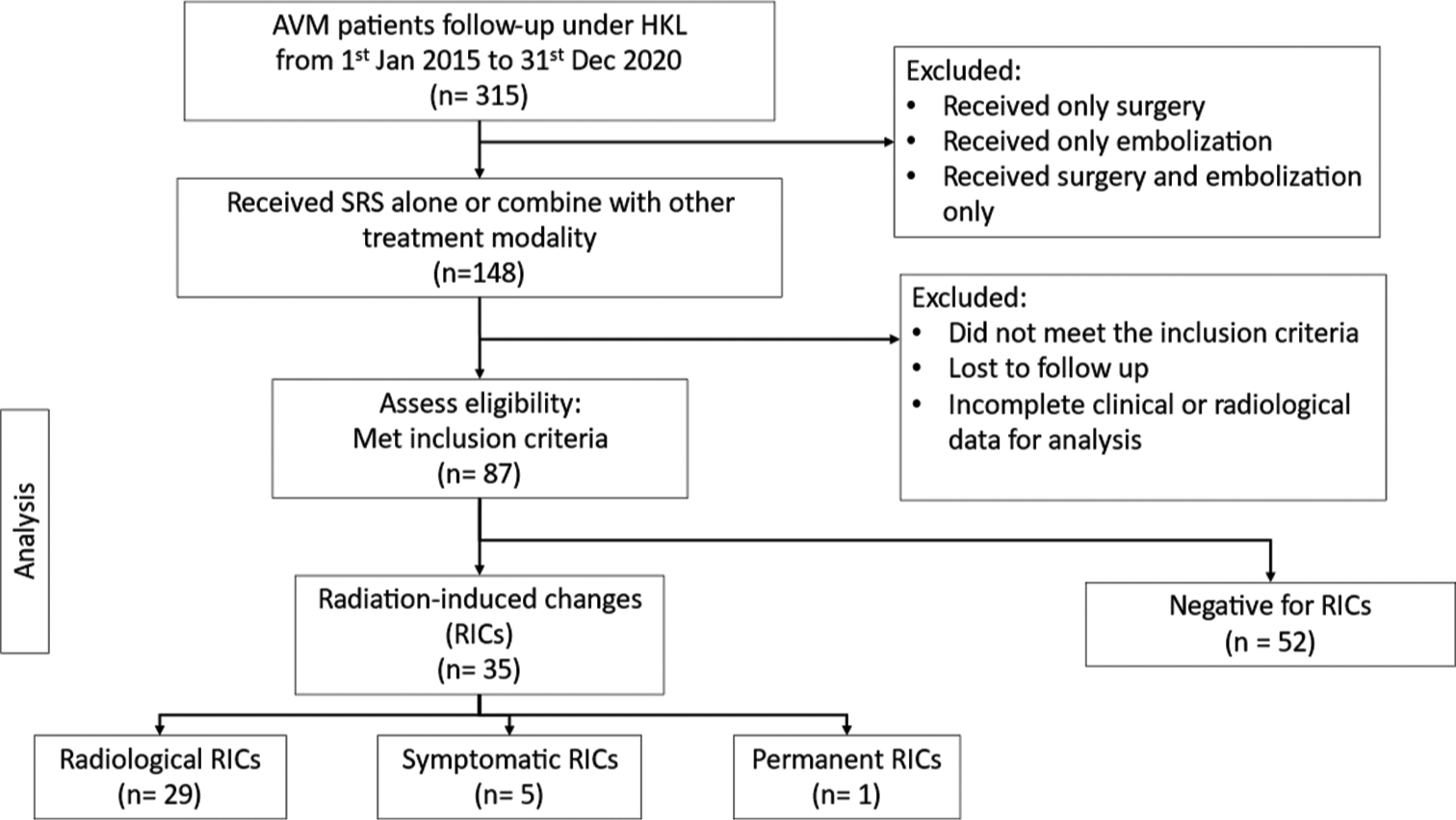
From January 1, 2015, to December 31, 2020, a total of 315 AVM patients were followed up at HKL. Among them, individuals who solely underwent surgery or embolization, as well as those who received both surgery and embolization, were excluded from the analysis, resulting in a cohort of 148 patients who received SRS alone or in combination with other treatment modalities. Following further exclusion of patients who did not meet the inclusion criteria, were lost to follow-up, or had incomplete clinical or radiological data, a total of 87 patients were deemed eligible for the final analysis [
DISCUSSION
Incidence and severity of RICs
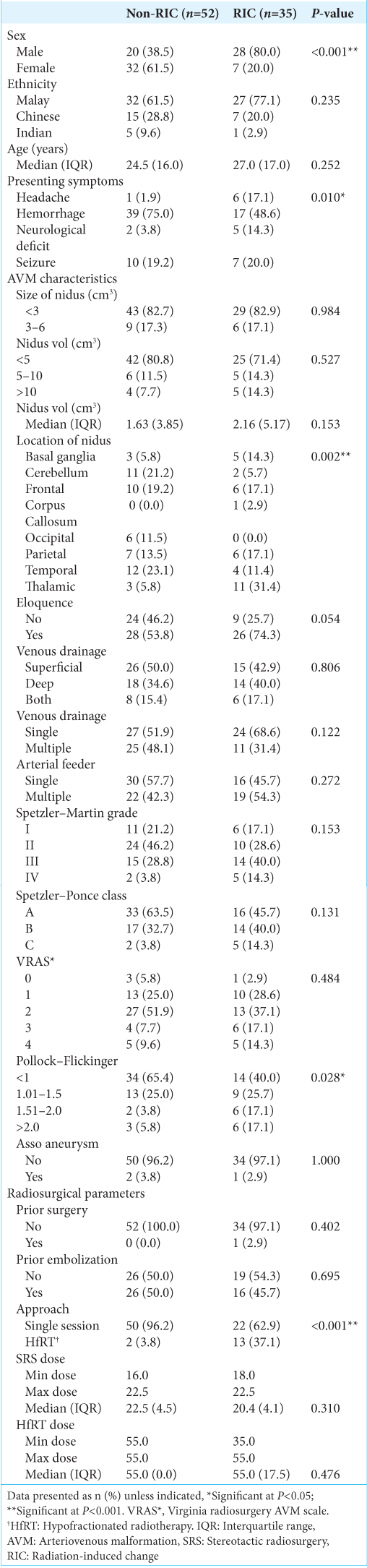
In this study, 35 patients (40.2%) developed RICs, categorized as radiological (33.3%), symptomatic (5.7%), and permanent (1.1%). When our findings were compared with those from Ilyas et al.’s meta-analysis, our rates closely aligned with their reported overall RIC rates of 35.5%, 9.2%, and 3.8% for radiological, symptomatic, and permanent changes, respectively.[
Risk factors of RICs
Brain eloquence and the Pollock–Flickinger score were identified as predictors, as detailed in
Another predictor in our study was the Pollock–Flickinger score. Previous studies have shown that a higher Pollock– Flickinger score is associated with an increased risk of RICs. [
Presenting symptom
Interestingly, a history of hemorrhage was inversely correlated with RIC development, possibly attributed to perinidal gliotic tissue acting as a protective barrier.[
Clinical implications
In
Limitations and future directions
While this study offered valuable insights into predictors and outcomes of RICs following SRS for AVMs, it was not without limitations. The retrospective nature and single-center design might have limited the generalizability of the findings, potentially introducing selection biases. In addition, the relatively small sample size and the study’s short follow-up period constrained our ability to assess long-term outcomes and the durability of treatment effects comprehensively. Future research should aim to conduct prospective, multi-center studies with larger patient cohorts and extended follow-up durations to validate and expand on our findings. Moreover, there is a need to explore the molecular and genetic mechanisms underlying RIC development to enhance predictive accuracy and develop targeted preventive strategies. Investigating the impact of emerging radiosurgical techniques and refining treatment protocols based on patient-specific factors will also be crucial in advancing the field and improving patient outcomes in AVM management.
CONCLUSION
This study provided crucial insights into RICs post-SRS for AVMs, demonstrating that 40.2% of patients developed RICs, a statistic that emphasized the need for personalized risk assessments in treatment planning. By identifying key predictors such as the Pollock–Flickinger score and eloquence of AVM location and assessing the impact of various radiosurgical techniques, this study enhanced our understanding of RICs, aligning with and expanding on the existing literature. The findings highlighted the complexity of RIC prediction and the significance of tailored patient care, offering valuable guidance for clinicians in optimizing treatment strategies and managing post-SRS outcomes. Through this enhanced understanding, the study contributed to improving clinical decision-making, aiming to refine SRS treatments and patient management to reduce RIC risks and improve overall treatment efficacy for AVM patients.
Ethical approval
The research/study was approved by the Institutional Review Board at the Medical Research and Ethics Committee, number NMRR ID-23-00369-LNJ (IIR), dated May 19, 2023.
Declaration of patient consent
Patient’s consent was not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
Acknowledgment
The authors would like to thank individuals who contributed to the study or manuscript preparation but who did not fulfill all the criteria of authorship.
References
1. Al-Shahi R, Bhattacharya JJ, Currie DG, Papanastassiou V, Ritchie V, Roberts RC. Prospective, population-based detection of intracranial vascular malformations in adults: The Scottish Intracranial Vascular Malformation Study (SIVMS). Stroke. 2003. 34: 1163-9
2. Andrade-Souza YM, Ramani M, Beachey DJ, Scora D, Tsao MN, terBrugge K. Liquid embolization material reduces the delivered radiation dose: A physical experiment. Acta Neurochir (Wien). 2008. 150: 161-4
3. China M, Vastani A, Hill CS, Tancu C, Grover PJ. Gamma Knife radiosurgery for cerebral arteriovenous malformations: A systematic review and meta-analysis. Neurosurg Rev. 2022. 45: 1987-2004
4. Cohen-Inbar O, Ding D, Chen CJ, Sheehan JP. Stereotactic radiosurgery for deep intracranial arteriovenous malformations, part 1: Brainstem arteriovenous malformations. J Clin Neurosci. 2016. 24: 30-6
5. Cohen-Inbar O, Ding D, Sheehan JP. Stereotactic radiosurgery for deep intracranial arteriovenous malformations, part 2: Basal ganglia and thalamus arteriovenous malformations. J Clin Neurosci. 2016. 24: 37-42
6. Daou BJ, Palmateer G, Wilkinson DA, Thompson BG, Maher CO, Chaudhary N. Radiation-induced imaging changes and cerebral edema following stereotactic radiosurgery for brain AVMs. Am J Neuroradiol. 2021. 42: 82-7
7. Ding D, Starke RM, Sheehan JP, editors. Radiosurgery for the management of cerebral arteriovenous malformations. Handbook of clinical neurology. Netherlands: Elsevier B.V.; 2017. 143: 69-83
8. Ding D, Yen CP, Starke RM, Xu Z, Sheehan JP. Effect of prior hemorrhage on intracranial arteriovenous malformation radiosurgery outcomes. Cerebrovasc Dis. 2015. 39: 53-62
9. Ding D, Yen CP, Xu Z, Starke RM, Sheehan JP. Radiosurgery for low-grade intracranial arteriovenous malformations. J Neurosurg. 2014. 121: 457-67
10. Ding D, Yen CP, Xu Z, Starke RM, Sheehan JP. Radiosurgery for primary motor and sensory cortex arteriovenous malformations: Outcomes and the effect of eloquent location. Neurosurgery. 2013. 73: 816-24
11. Fliciunger JC, Kondziolka D, Pollock BE, Mapz AH, Dade Lunsford L. Complications from arteriovenous malformation radiosurgery: Multivariate analysis and risk modelling. Int J Radiat Oncol Biol Phys. 1997. 38: 485-90
12. Flickinger JC, Kondziolka D, Lunsford LD, Kassam A, Phuong LK, Liscak R. Clinical investigation central nervous system development of a model to predict permanent symptomatic postradiosurgery injury for arteriovenous malformation patients. Int J Radiat Oncol Biol Phys. 2000. 46: 1143-8
13. Flickinger JC, Kondziolka D, Maitz AH, Lunsford LD. An analysis of the dose-response for arteriovenous malformation radiosurgery and other factors affecting obliteration. Radiother Oncol. 2002. 63: 347-54
14. Flickinger JC, Lunsford LD, Kondziolka D, Maitz AH, Epstein AH, Simons SR. Radiosurgery and brain tolerance: An analysis of neurodiagnostic imaging changes after gamma knife radiosurgery for arteriovenous malformations. Int J Radiat Oncol Biol Phys. 1992. 23: 19-26
15. Flickinger JC, Pollock BE, Kondziolka D, Lunsford LD. A dose-response analysis of arteriovenous malformation obliteration after radiosurgery. Int J Radiat Oncol Biol Phys. 1996. 36: 873-9
16. Friedman WA, Bova FJ, Mendenhall WM. Linear accelerator radiosurgery for arteriovenous malformations: the relationship of size to outcome. J Neurosurg. 1995. 82: 180-9
17. Fukuda K, Majumdar M, Masoud H, Nguyen T, Honarmand A, Shaibani A. Multicenter assessment of morbidity associated with cerebral arteriovenous malformation hemorrhages. J Neurointerv Surg. 2017. 9: 664-8
18. Graffeo CS, Link MJ, Stafford SL, Garces YI, Foote RL, Pollock BE. More II it than meets the eye: Outcomes after single-fraction stereotactic radiosurgery in a case series of low-grade arteriovenous malformations. Oper Neurosurg. 2020. 18: 136-44
19. Graffeo CS, Sahgal A, De Salles A, Fariselli L, Levivier M, Ma L. Stereotactic radiosurgery for Spetzler-Martin Grade I and II arteriovenous malformations: International society of stereotactic radiosurgery (ISRS) practice guideline. Neurosurgery. 2020. 87: 442-52
20. Hayhurst C, Monsalves E, van Prooijen M, Cusimano M, Tsao M, Menard C. Pretreatment predictors of adverse radiation effects after radiosurgery for arteriovenous malformation. Int J Radiat Oncol Biol Phys. 2012. 82: 803-8
21. Ilyas A, Chen CJ, Ding D, Buell TJ, Raper DM, Lee CC. Radiation-induced changes after stereotactic radiosurgery for brain arteriovenous malformations: A systematic review and meta-analysis. Clin Neurosurg. 2018. 83: 365-76
22. Jiang Z, Zhang X, Wan X, Wei M, Liu Y, Ding C. Efficacy and safety of combined endovascular embolization and stereotactic radiosurgery for patients with intracranial arteriovenous malformations: A systematic review and meta-analysis. Biomed Res Int. 2021. 2021: 6686167
23. Kalimo H, Kase M, Haltia M, editors. Greenfield’s neuropathology. New York: Oxford University Press; 1997. p.
24. Kano H, Flickinger JC, Tonetti D, Hsu A, Yang H, Flannery TJ. Estimating the risks of adverse radiation effects after gamma knife radiosurgery for arteriovenous malformations. Stroke. 2017. 48: 84-90
25. Katsura M, Sato J, Akahane M, Furuta T, Mori H, Abe O. Recognizing radiation-induced changes in the central nervous system: Where to look and what to look for. Radiographics. 2021. 41: 224-48
26. Kim MJ, Chang KW, Park SH, Chang WS, Chang JH, Chang JW. Predictive factors of radiation-induced changes following single-session gamma knife radiosurgery for arteriovenous malformations. J Clin Med. 2021. 10: 2186
27. Kobayashi T, Kida Y, Tanaka T, Yoshida K, Mori Y, Ohosuka K. Eloquent areas in the gamma knife treatment of arteriovenous malformations of the brain. Jpn J Neurosurg. 1999. 8: 385-94
28. Lee CC, Chen CJ, Ball B, Schlesinger D, Xu Z, Yen CP. Stereotactic radiosurgery for arteriovenous malformations after Onyx embolization: A case-control study. J Neurosurg. 2015. 123: 126-35
29. Martin NA, Vinters HV, editors. Neurovascular surgery. New York: McGraw-Hill; 1995. p.
30. Nataf F, Schlienger M, Bayram M, Ghossoub M, George B, Roux FX. Microsurgery or radiosurgery for cerebral arteriovenous malformations? A study of two paired series. Neurosurgery. 2007. 61: 39-50
31. Nguyen BT, Huynh CT, Nguyen TM, Nguyen VT, Karras CL, Huynh-Le P. Gamma knife radiosurgery for brain arteriovenous malformations: A 15-year single-center experience in southern Vietnam. World Neurosurg. 2022. 163: 71-9
32. Oermann EK, Ding D, Yen CP, Starke RM, Bederson JB, Kondziolka D. Effect of prior embolization on cerebral arteriovenous malformation radiosurgery outcomes. Neurosurgery. 2015. 77: 406-17
33. Peciu-Florianu I, Leroy HA, Drumez E, Dumot C, Aboukaïs R, Touzet G. Radiosurgery for unruptured brain arteriovenous malformations in the pre-ARUBA era: Long-term obliteration rate, risk of hemorrhage and functional outcomes. Sci Rep. 2020. 10: 21427
34. Pollock BE, Brown RD. Use of the Modified Rankin Scale to assess outcome after arteriovenous malformation radiosurgery. Neurology. 2006. 67: 1630-4
35. Pollock BE, Flickinger JC. A proposed radiosurgery-based grading system for arteriovenous malformations. J Neurosurg. 2002. 96: 79-85
36. Pożarowszczyk N, Kurkowska-Jastrzębska I, SarzyńskaDługosz I, Nowak M, Karliński M. Reliability of the modified Rankin Scale in clinical practice of stroke units and rehabilitation wards. Front Neurol. 2023. 14: 1064642
37. Russell D, Peck T, Ding D, Chen CJ, Taylor DG, Starke RM. Stereotactic radiosurgery alone or combined with embolization for brain arteriovenous malformations: A systematic review and meta-analysis. J Neurosurg. 2018. 128: 1338-48
38. Schlienger M, Atlan D, Lefkopoulos D, Merienne L, Touboul E, Missir O. Linac radiosurgery for cerebral arteriovenous malformations: Results in 169 patients. Int J Radiat Oncol Biol Phys. 2000. 46: 1135-42
39. Sethi A, Chee K, Chatain GP, Wittenberg B, Seinfeld J, Milgrom S. Time-dosed stereotactic radiosurgery for the treatment of cerebral arteriovenous malformations: An early institution experience and case series. Neurosurg Pract. 2023. 4: e00056
40. Shah SN, Shah SS, Kaki P, Satti SR, Shah SA. Efficacy of dose-escalated hypofractionated radiosurgery for arteriovenous malformations. Cureus. 2024. 16: e52514
41. Shtraus N, Schifter D, Corn BW, Maimon S, Alani S, Frolov V. Radiosurgical treatment planning of AVM following embolization with Onyx: Possible dosage error in treatment planning can be averted. J Neurooncol. 2010. 98: 271-6
42. Stapf C, Mast H, Sciacca RR, Berenstein A, Nelson PK, Gobin YP. The New York Islands AVM study. Stroke. 2003. 34: e29-33
43. Starke RM, Kano H, Ding D, Lee JY, Mathieu D, Whitesell J. Stereotactic radiosurgery for cerebral arteriovenous malformations: Evaluation of long-term outcomes in a multicenter cohort. J Neurosurg. 2017. 126: 36-44
44. Valle RD, Zenteno M, Jaramillo J, Lee A, De Anda S. Definition of the key target volume in radiosurgical management of arteriovenous malformations: A new dynamic concept based on angiographic circulation time. J Neurosurg. 2008. 109: 41-50
45. van den Berg R, Buis DR, Lagerwaard FJ, Nijeholt GJ, Vandertop WP. Extensive white matter changes after stereotactic radiosurgery for brain arteriovenous malformations. Neurosurgery. 2008. 63: 1064-70
46. Voshart DC, Wiedemann J, van Luijk P, Barazzuol L. Regional responses in radiation-induced normal tissue damage. Cancers (Basel). 2021. 13: 367
47. Yan D, Chen Y, Li Z, Zhang H, Li R, Yuan K. Stereotactic radiosurgery with vs. without prior embolization for brain arteriovenous malformations: A propensity score matching analysis. Front Neurol. 2021. 12: 752164
48. Yang HC, Wu HM, Peng SJ, Lee CC, Chen YW, Kuan AS. The irradiated brain volume within 12 Gy is a predictor for radiation-induced changes after stereotactic radiosurgery in patients with unruptured cerebral arteriovenous malformations. Int J Radiat Oncol Biol Phys. 2021. 111: 785-93
49. Yen CP, Matsumoto JA, Wintermark M, Schwyzer L, Evans AJ, Jensen ME. Radiation-induced imaging changes following Gamma Knife surgery for cerebral arteriovenous malformations. J Neurosurg. 2013. 118: 63-73
50. Zhu D, Li Z, Zhang Y, Fang Y, Li Q, Zhao R. Gamma knife surgery with and without embolization for cerebral arteriovenous malformations: A systematic review and meta-analysis. J Clin Neurosci. 2018. 56: 67-73