- Hand and Microsurgery Center, The Second Affiliated Hospital of Harbin Medical University, Nangang, Harbin 150081, China
- Department of Stem Cell Biology, School of Medicine, Konkuk University, Seoul 10100, Korea
- Department of MR Diagnosis, The Second Affiliated Hospital of Harbin Medical University, Nangang, Harbin 150081, China
- Department of Anesthesia, The Second Affiliated Hospital of Harbin Medical University, Nangang, Harbin 150081, China
- Department of Pharmacology, Harbin Medical University, Nangang, Harbin 150081, China
- Department of Laboratory Animal Medicine, College of Veterinary Medicine, Seoul National University, Seoul 10100, Korea
- Department of Neurology, The Second Affiliated Hospital of Harbin Medical University, Nangang, Harbin 150081, China
DOI:10.25259/SNI-73-2019
Copyright: © 2019 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Shuai Ren, Zehan Liu, C. Yoon Kim, Kuang Fu, Qiong Wu, Liting Hou, Linlin Sun, Jian Zhang, Qing Miao, Jin Kim, Vincenzo Bonicalzi, Xiangchen Guan, Mingzhe Zhang, Weihua Zhang, Junfeng Xu, Sergio Canavero, Xiaoping Ren. Reconstruction of the spinal cord of spinal transected dogs with polyethylene glycol. 26-Mar-2019;10:50
How to cite this URL: Shuai Ren, Zehan Liu, C. Yoon Kim, Kuang Fu, Qiong Wu, Liting Hou, Linlin Sun, Jian Zhang, Qing Miao, Jin Kim, Vincenzo Bonicalzi, Xiangchen Guan, Mingzhe Zhang, Weihua Zhang, Junfeng Xu, Sergio Canavero, Xiaoping Ren. Reconstruction of the spinal cord of spinal transected dogs with polyethylene glycol. 26-Mar-2019;10:50. Available from: http://surgicalneurologyint.com/surgicalint-articles/9233/
Abstract
Background: Our study shows that a membrane sealant/fiber fusogen polyethylene glycol (PEG) applied immediately on a sharp section of the spinal cord can mend the cord and lead to exceptional levels of motor recovery, with some animals almost normal.
Materials and Methods: Before deploying such technology in man, long-term data in large mammals that exclude delayed complications (e.g., central pain), confirm the stability of motor recovery, and provide histological evidence of fiber regrowth are necessary. Here, we provide such evidence in dogs followed up over 6 months and in 2 cases up to 1 year along with imaging and histologic data.
Results: We show that dogs whose dorsal cord has been fully transected recover locomotion after immediate treatment with a fusogen (PEG). No pain syndrome ensued over the long term. Diffusion tensor imaging magnetic resonance and histological, including immunohistochemical, data confirmed the re-establishment of anatomical continuity along with interfacial axonal sprouting.
Conclusions: This study proves that a form of irreversible spinal cord injury (SCI) can effectively be treated and points out a way to treat SCI patients.
Keywords: Polyethylene glycol, spinal cord fusion, spinal cord injury
INTRODUCTION
We recently showed how polyethylene glycol (PEG), a fusogen[
MATERIALS AND METHODS
All animal procedures were reviewed and approved by the Institutional Animal Care and Use Committee of Harbin Medical University (HMUIRB-2008-06) and the Institute of Laboratory Animal Science of China (A5655-01) and were in accordance with Directive 2010/63/EU of the European Parliament. Female beagles (8 kg) were used. The experimental group (PEG) consisted of 7 animals; 5 controls were treated with 0.9% NaCl. Spinal cord transection (SCT) at T10 was performed under general anesthesia. Animals were randomized to receive either 0.9% NaCl (2 mL) or PEG-600 (2 mL) applied topically to the site of SCT through a syringe and left in situ at the point of transection.[
Neuroimaging
All animals were subjected to magnetic resonance imaging (MRI) and diffusion tensor imaging (DTI) using a 3.0 T MRI system (Achieva 3, Philips, Amsterdam, and The Netherlands) in the prone position. Sagittal, T2-weighted, fast spin-echo (TR = 1700 ms; TE = 100 ms; slice thickness = 3 mm; slice gap = 0.1; and NSA = 4), and axial single-shot echo-planar DTI (TR = 6100 ms; TE = 93 ms; voxel size = 2 mm × 2 mm; slice thickness = 2 mm; slice gap = 0; NSA = 2; and diffusion direction number = 15) sequences were acquired twice at 2 and 4 weeks postoperatively in all animals and then at 180 days.
Histologic assessment
After 6 months, all surviving beagles, except two from the PEG group, were anesthetized and perfused with 0.9% NaCl solution and 4% paraformaldehyde. Immediately thereafter, the T10 spinal cord was surgically accessed and the treated cord extracted en bloc by sectioning 2 cm above and below the point of fusion. The bloc was immersed in 4% paraformaldehyde for 24 h, followed by paraffin embedding.
Hematoxylin-Eosin (HE) and Chromotropic acid 2R-Brilliant Green (C-2R-G) stainings were carried out employing commercially available kits (Leagene, Beijing, PR China): C-2R-G stains myelin in red and the acellular extracellular matrix in green. All cords were studied on sagittal and transverse slices above, at and below the site of transection.
For HE staining, 7 µm-thick sagittal and coronal sections were dewaxed, hydrated and then placed directly into xylene twice for 10’, and then in alcohol at decreasing concentrations for 5’ each (100%, 95%, 85%, and 70%). The sections were rinsed in peroxidase blocking soluiton (PBS) 3 times for 5’, stained with hematoxylin for 10’ and then rinsed in distilled water; finally, the sections were differentiated in a 1% HCl solution and then dipped in distilled water. Sections were then stained with eosin for 3’ and then dehydrated in gradient ethanol; subsequently, the sections were placed into xylene twice for 5’. For C-2R-G staining, 7-μm thick sections were dewaxed and hydrated, then passage into xylene for 20’, 100% alcohol for 1’, and 95% alcohol for 5’. Afterward, the sections were stained with C-2R-G for 10’ followed by a triple rinse in 0.2% glacial acetic acid and counterstained in 0.5% brilliant green glacial acetic acid solution for 10’. Subsequently, sections were rinsed in distilled water for 2’, dehydrated in gradient ethanol, and treated with xylene twice for 5’.
All sections were visualized with an Olympus IX73 (Tokyo, Japan) optical microscope. The Image-Pro-Plus (Media Cybernetics, Rockville, MD, USA) software was employed for image analysis.
Immunohistochemistry
Longitudinal sections of spinal cord tissue cut at 10 µm were mounted onto silanized glass slides. Slides were air-dried for 15 min and washed in PBS before blocking in 10% normal bovine serum and 0.1% Triton X-100 in PBS at room temperature. Primary antibodies diluted in Dako antibody diluent (Dako, Denmark) were applied overnight at 4°C in a humidified chamber. Primary antibodies included anti-serotonin (5-HT; 1:1000, Immunostar, WI, USA) and anti-neurofilament 200 (NF200) (1:400; Sigma, USA). Slides were washed and secondary antibodies diluted in PBS applied for 1 h at room temperature – Alexa Fluor 488 or 594 conjugated IgG (1:2000, Molecular Probes, USA). Slides were washed in PBS and mounted with a cover-slip using VECTASHIELD (Vector Lab. the US). Images were acquired using a fluorescence microscope fitted with a digital camera system (Nikon, Japan) and routed to a Windows PC for quantitative analyses using Adobe Photoshop CS5 software. Attention was given to ensure identical settings for fluorescence exposure, amplifier gain and cut off across all images.
Statistical analysis
Datametrics for DTI were calculated by the machine software. Histologic indices (vacuolization, scarring, and myelin ratios) were calculated with GraphPad Prism 5 (LaJolla, CA, USA).
RESULTS
All 12 animals survived the operation. Initially, all animals had normal initial cBBB scores [
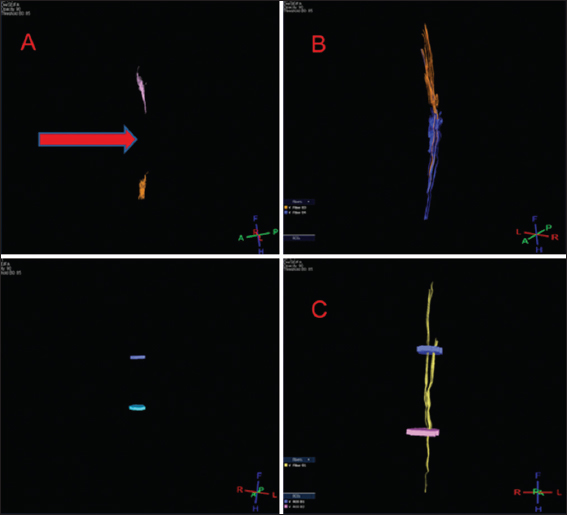
Tractography
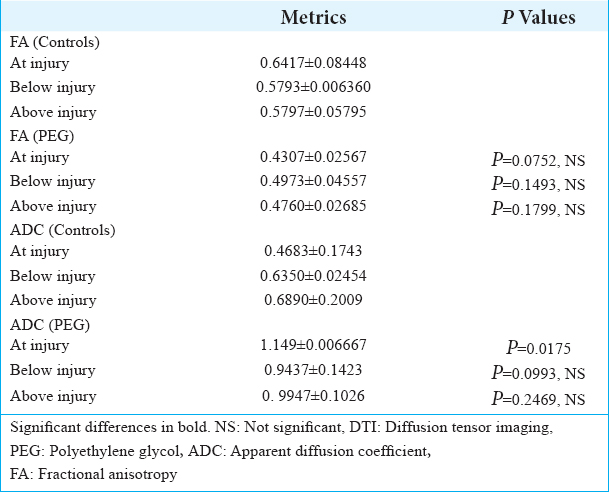
DTI showed tissue re-establishment of anatomical continuity in PEG-treated animals as opposed to controls [
Histology and immunohistochemistry
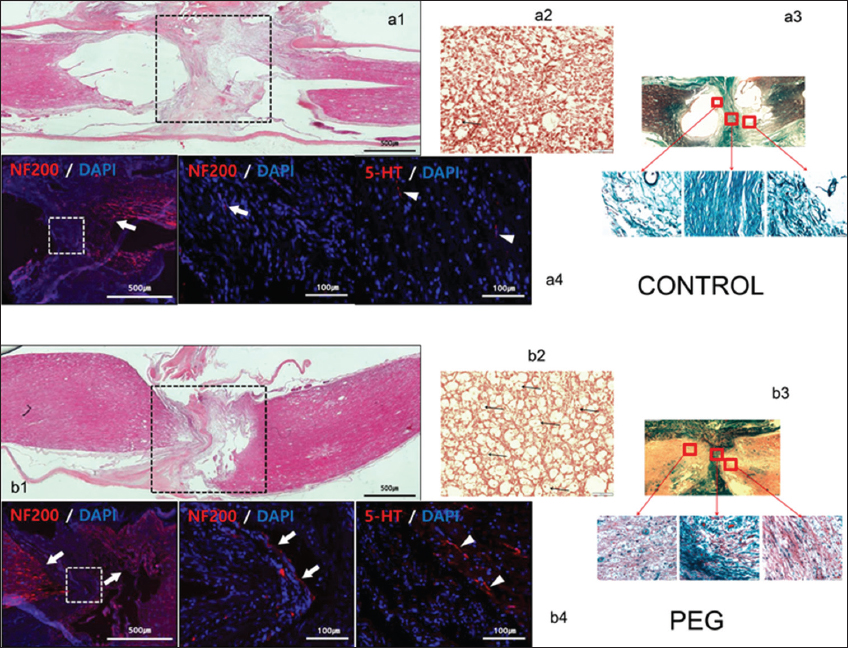
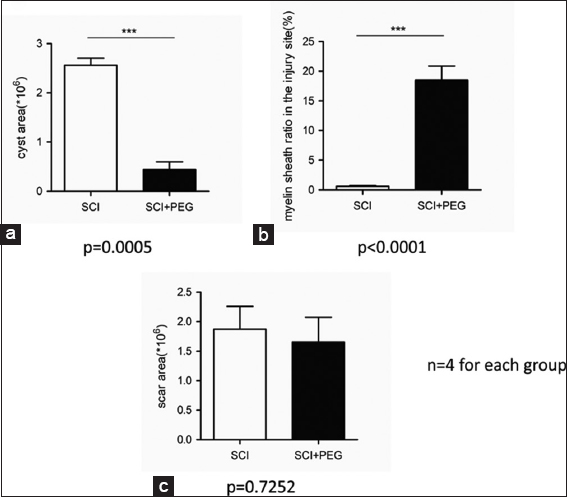
On HE stained sections (sagittal and coronal), treated and untreated cords differed dramatically: vacuolization due to tissue injury (cysts) was minimal in treated cords [
Figure 2
Histology and immunohistochemistry. Controls: a1: hematoxylin-eosin (HE) sagittal view of the cord: notice poor take-up of the stain by interface tissues. a2: chromotropic acid 2R-Brilliant Green (C-2R-G) staining of an axial slice showing widespread Wallerian degeneration. a3: C-2R-G stained sections above, at and below injury level: notice absence of axons across the interface. a4: neurofilament 200 (NF200) DAPI and 5HT DAPI sections. Notice near absence of regrowing fibers across the interface. Polyethylene glycol: b1: HE sagittal view of the cord: notice bright coloring as stains are taken up by treated tissues. b2: C-2R-G staining of an axial slice showing widely conserved axons at the interface (arrows). b3: C-2R-G stained sections above and below injury level: notice the abundance of axons across the interface. b4: NF200 DAPI and 5HT DAPI sections. Notice regrowing fibers across the interface.
DISCUSSION
This study shows that a membrane sealant/fiber fusogen (PEG) applied immediately on a sharp section of the spinal cord can re-establish anatomical continuity (and lead to behavioral recovery).
Sharpness of the transection is a key factor for successful axonal regeneration. An extremely sharp transection produces edema-free lesions, without cysts not scars, whereas a relatively blunt An extremely sharp transection followed by scars and cysts around the lesions.[
Both neuroimaging and histology confirm the neuroprotective effects on cells at the level of the section and regrowth of fibers across the fusion interface. This regrowth is focused on a cellular, short-fiber, and gray matter-based network of interneurons extending from the brainstem to the spinal cord -and inputed by fibers from cortical motor areas- that simultaneously conveys commands to motoneurons (Cortico-Truncoreticulo-Propriospinal Pathway – C-TRPS); it also embeds and links the Central Pattern Generators located in the cervical and lumbar cord responsible for the execution of motor programs, including gait.[
Given the excellent recovery, the sprouting process in the TRPS does not appear to be haphazard, with disruptive misalignments. In any case, circuital readjustment in the spinal cord and within upper CNS stations is known to play a key role in recovery from spinal cord injuries (SCI).[
PEG does not appear to act via interfering with the scarring process, as shown by the nonsignificant difference between the two groups. PEG is applied immediately after SCT: no astrocytic scar is in place to hinder the process since a scar only becomes visible after about 1 week of injury. The astrocytic scar might actually promote axon regrowth in the early stages of SCI: it is only past the subacute stage that the scar slowly becomes nonpermissive.[
CONCLUSIONS
We have shown that the paralysis following full section of the spinal cord can be reversed by immediate topical application of a fusogen at the point of the section: recovery is due to axonal sprouting across the fusion interface.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
The authors are supported by the National Natural Science Foundation of China (81470425), the Wu Liande Foundation of Harbin Medical University (Wld-qn1414), and the Harbin Science and Technology Bureau (2014RFXYJ023).
References
1. Abdou SA, Henderson PW. Fusogens: Chemical agents that can rapidly restore function after nerve injury. J Surg Res. 2019. 233: 36-40
2. Canavero S, Ren X, Kim CY, Rosati E. Neurologic foundations of spinal cord fusion (GEMINI). Surgery. 2016. 160: 11-9
3. Canavero S, Ren X, Kim CY. Reconstructing the severed spinal cord. Surg Neurol Int. 2017. 8: 285-
4. Canavero S, Ren X. Houston, GEMINI has landed: Spinal cord fusion achieved. Surg Neurol Int. 2016. 7: S626-8
5. Isa T. The brain is needed to cure spinal cord injury. Trends Neurosci. 2017. 40: 625-36
6. Kim CY, Oh H, Ren X, Canavero S. Immunohistochemical evidence of axonal regrowth across polyethylene glycol-fused cervical cords in mice. Neural Regen Res. 2017. 12: 149-50
7. Kim CY. PEG-assisted reconstruction of the cervical spinal cord in rats: Effects on motor conduction at 1h. Spinal Cord. 2016. 54: 910-2
8. Liu Z, Ren S, Fu K, Wu Q, Wu J, Hou L. Restoration of motor function after operative reconstruction of the acutely transected spinal cord in the canine model. Surgery. 2018. 163: 976-83
9. Lu X, Perera TH, Aria AB, Callahan LA. Polyethylene glycol in spinal cord injury repair: A critical review. J Exp Pharmacol. 2018. 10: 37-49
10. Ren S, Liu ZH, Wu Q, Fu K, Wu J, Hou LT. Polyethylene glycol-induced motor recovery after total spinal transection in rats. CNS Neurosci Ther. 2017. 23: 680-5
11. Rolls A, Shechter R, Schwartz M. The bright side of the glial scar in CNS repair. Nat Rev Neurosci. 2009. 10: 235-41
12. Xia Y, Chen D, Xia H, Liao Z, Tang W, Yan Y. Serotonergic projections to lumbar levels and its plasticity following spinal cord injury. Neurosci Lett. 2017. 649: 70-7
13. Yoshida Y, Kataoka H, Kanchiku T, Suzuki H, Imajyo Y, Kato H. Transection method for shortening the rat spine and spinal cord. Exp Ther Med. 2013. 5: 384-8






Harry S. Goldsmith, MD
Posted April 4, 2019, 2:46 pm
It has been shown that transected mammalian axons have the capacity to regrow and develop new connections with nerve tissue distal to the transection site. Such axons display orderly neuroelectrical activity and functional return.
Dr. Ren and his associates have reported that a fusugen agent (polyethylene glycol, also known as peg) when placed directly between the cut ends of a divided spinal cord may prevent paralysis “due to axonal sprouting across the fusugen (peg) interface.” However, it is not stated that these axons travel to the distal spinal cord, an action which is necessary if paralysis is to be prevented.
Ramon y Cajal has claimed that the reason paralysis follows a spinal cord injury (sci) is that the injury results in a spinal cord scar which prevents axons from penetrating through the scar to distal neural connections.
Dr. Ren’s paper did not refer to the space that results following the excision of a spinal cord scar. Placing the cut ends of a severed spinal cord together, separated by peg, will require spinal column surgery to close the gap that results from the sci scar removal. A procedure that is known to eliminate the spinal cord gap following the excision of a spinal cord scar can be accomplished by placing collagen in the gap between the cut ends of the divided spinal cord. A vascularized omental graft is then placed directly on the collagen bridge to supply blood to the collagen. It has been shown that axons penetrate through the collagen bridge and progress down to the distal spinal cord at the rate of 1 mm per day. Axons have been identified 90 mm from the collagen bridge three months following surgery. This procedure has resulted in functional return in both animals and man (1, 2, 3, 4, 5).
References
1. Goldsmith HS, de la Torre JC. Axonal regeneration after spinal cord transection and reconstruction. Brain Research, 589: 217-224, 1992
2. Goldsmith HS, Fonseca A JR, Porter J. Spinal cord separation: MRI evidence of healing after omental-collagen reconstruction. Neurol Res 27: 115-123, 2005
3. Goldsmith HS, Application of the omentum to the brain and spinal cord. In The Omentum: Basic Research and Clinical Application. Goldsmith HS, Editor. Woodbury, CT, Cine-Med Publishing. Chapter 3, 37-52, 2010.
4. Goldsmith HS. Omental transposition and spinal surgery: emphasis on revascularization and scar prevention. In: Spinal Surgery: Techniques and Management. Benzel EC, Editor. Philadelphia, Elsevier Saunders. Chapter 121, 1183-1187, 2012
5. Goldsmith HS. Improvement by the omentum in spinal cord injuries. Clinics in Surgery, July 18, 1-5, 2018
Aliaksei Charapko m.d.
Posted May 19, 2019, 1:18 pm
These research give us hope that we will be able to cure difficult neurological diseases such as amiotrophical sclerosis or posttraumatical paralysis through operations with using PEG to recover axonal activity. The comparison with placebi shiws that it works
Cisnenegro
Posted March 20, 2021, 4:48 pm
Ahora solo nos queda aprender a diseñar neuronas con mayor capacidad de resistir la falta de oxígeno, similar a lo que consiguen en heterocephalus glaber.