- Department of Neurologic Surgery, Mayo Clinic, Rochester, Minnesota, USA
- Department of Neurology, Mayo Clinic, Rochester, Minnesota, USA
Correspondence Address:
William E. Krauss
Department of Neurologic Surgery, Mayo Clinic, Rochester, Minnesota, USA
DOI:10.4103/2152-7806.191022
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Graffeo CS, Puffer RC, M. Wijdicks EF, Krauss WE. Delayed cerebral infarct following anterior cervical diskectomy and fusion. Surg Neurol Int 21-Sep-2016;7:86
How to cite this URL: Graffeo CS, Puffer RC, M. Wijdicks EF, Krauss WE. Delayed cerebral infarct following anterior cervical diskectomy and fusion. Surg Neurol Int 21-Sep-2016;7:86. Available from: http://surgicalneurologyint.com/surgicalint_articles/delayed-cerebral-infarct-following-anterior-cervical-diskectomy-fusion/
Abstract
Background:Ischemic stroke following anterior cervical diskectomy and fusion (ACDF) is an exceedingly rare complication. There are only three previous cases focusing on this problem in the literature; here, we present the fourth case.
Case Description:A patient, cared for at an outside institution, developed a delayed ischemic stroke 3 days following an ACDF. This complication was attributed to carotid manipulation precipitating vascular injury in the setting of multiple comorbid vascular and coagulopathic risk factors, including previously undiagnosed carotid atherosclerosis, a prior history of pulmonary embolus requiring Warfarin anticoagulation (held perioperatively), acute dehydration, and atrial fibrillation.
Conclusions:This case demonstrates the importance of focused history and examination in appropriate patients prior to ACDF, with special consideration given to the significance of age, comorbidities including coagulopathy and arrhythmia, and potential underlying vascular disease as markers for increased risk of perioperative thrombotic stroke associated with carotid manipulation. Patients at higher risk warrant comprehensive preoperative assessment, including medical evaluation, carotid imaging, and consideration for alternative surgical approaches.
Keywords: Anterior cervical diskectomy and fusion, carotid disease, cerebral infarct, screening
BACKGROUND
Cerebral infarct due to carotid injury is a very rare complication of anterior cervical diskectomy and fusion (ACDF) surgery; there are only three prior cases reported in the literature. In each of these cases, the patient awakened from anesthesia with a new neurologic deficit, having sustained a cerebral infarct. Subsequent investigations revealed a wide range of underlying stroke etiologies, including prolonged retraction compressing the carotid artery, a congenitally abnormal Circle of Willis, and hypotension in the setting of atherosclerosis.[
CASE DESCRIPTION
A 61-year-old man with a history of PE fibrillation requiring warfarin anticoagulation and hypertension presented to an outside hospital with 8 months of progressive neck pain and right upper extremity pain, which had worsened to the point of awakening him at night. Multiple attempts at physical therapy, chiropractic manipulation, and other nonoperative modalities had failed to relieve this patient's pain. His neurologic examination demonstrated 3/5 strength in the right triceps and pectoralis major, with obvious muscle atrophy; there were no signs of myelopathy, hyperreflexia, pathologic reflexes, or gait disturbance. Records from this community practice—including but not limited to preoperative assessment, imaging studies, and operative report—were reviewed extensively following his ultimate transfer to our facility and are presented here in a retrospective review.
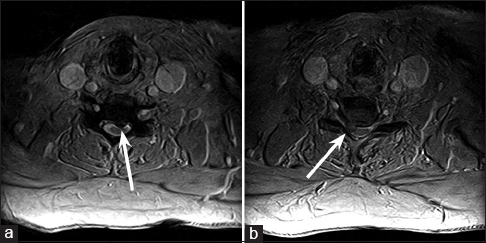
Magnetic resonance imaging (MRI) of the cervical spine demonstrated a moderate near-midline left herniation of the C5-C6 intervertebral disk compressing the cervical cord, as well as a large right paramedian herniation of the C6-C7 intervertebral disk compressing the right C7 nerve root [Figure
Figure 1
T2-weighted axial magnetic resonance imaging of the cervical spine through the C5-C6 and C6-C7 levels, demonstrating a moderate near-midline left herniation of the C5-C6 intervertebral disk compressing the cervical cord (a), as well as a large right paramedian herniation of the C6-C7 intervertebral disk compressing the right C7 nerve root (b)
Warfarin anticoagulation was held for 10 days preoperatively. Surgery included a routine right-sided exposure to perform a two-level ACDF at C5-C6 and C6-C7, which was completed using the Smith–Robinson technique. Notably, a self-retaining radiolucent retractor system was placed without difficulty and was periodically relaxed throughout the case; a monitored endotracheal tube was also used as surveillance for recurrent laryngeal nerve injury, and no abnormalities were noted.
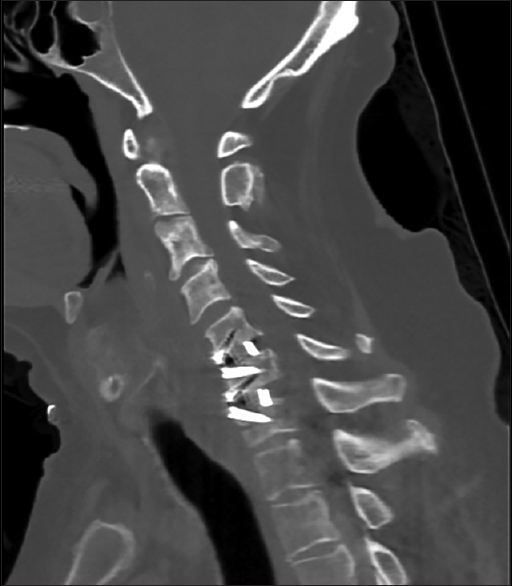
There were no intraoperative complications, and careful inspection of the carotid artery, jugular vein, and surrounding structures prior to closure revealed no abnormalities. The wound was closed in the normal anatomic layers, and the patient awoke from general anesthesia without neurologic deficit and recovered uneventfully. Postoperative radiographs demonstrated normal alignment of the cervical spine with appropriate positioning of the fusion construct [
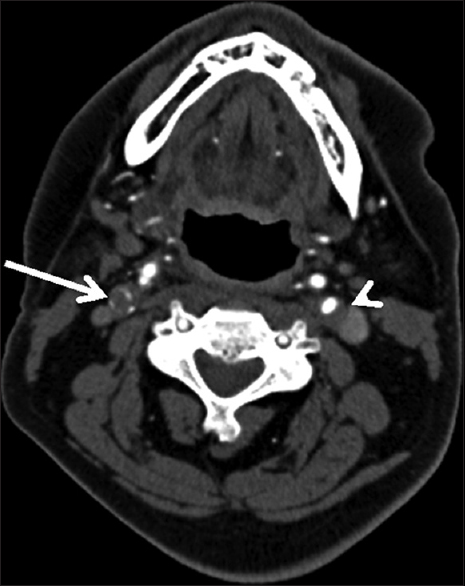
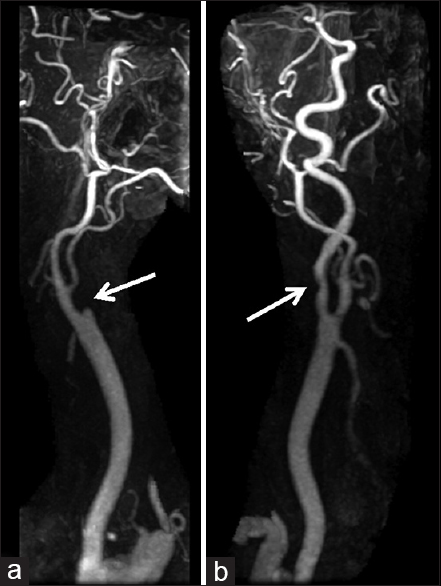
Three days after the discharge, the patient experienced multiple episodes of acute-onset large-volume diarrhea and vomiting overnight. The following morning, he had difficulty using his left arm and leg, with associated slurring of his speech. He was urgently transported to a local emergency department, where computed tomography (CT) of the head demonstrated subtle early ischemic changes involving the right hemisphere, including the insula and deep white matter of the frontal lobe (not shown). CT angiogram (CTA) of the head and neck revealed near-complete occlusion of the right internal carotid artery and greater than 50% stenosis of the left internal carotid artery, with otherwise normal arterial anatomy without evidence of dissection [
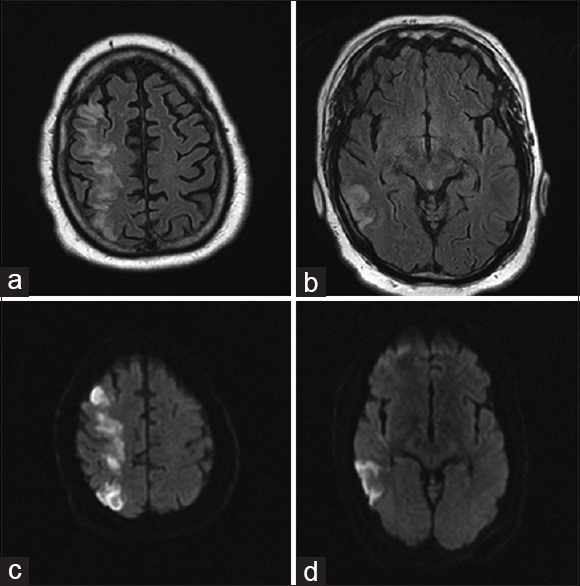
On arrival, National Institute of Health Stroke Scale score was 14 with right gaze preference and left homonymous hemianopsia, lower facial droop, left hemiplegia, sensory loss, and hemineglect. The surgical site was clean, dry, and intact, with no signs concerning for hematoma, infection, mass, or other compressive process. MRI/MRA of the head and neck showed greater than 50% occlusion of the left internal carotid artery without evidence of dissection, complete occlusion of the right internal carotid artery at its origin with slow reconstitution at the petrous level, slow intracranial flow distally indicating insufficient collateral flow, and extensive diffusion restriction and fluid-attenuated inversion recovery changes in a typical watershed distribution throughout the right hemisphere [Figures
Electrocardiogram was noteworthy for new-onset atrial fibrillation, with ventricular rate to 130, which was medically managed using diltiazem and metoprolol. Echocardiogram showed no evidence of cardiac thrombus or patent foramen ovale. Ultrasound of the lower extremities revealed an acute-on-chronic deep venous thrombosis of the right popliteal vein. Follow-up imaging with CTA of the chest showed multiple acute emboli, with early pulmonary infarction in the periphery of the right mid-lung, without evidence of right heart strain. Given the contraindication to therapeutic anticoagulation, an inferior vena cava filter was placed. Hematology and coagulation studies showed a normal international normalized ratio, partial thromboplastin time, and bleeding time, with no evidence of an underlying heritable coagulopathy.
The patient was observed overnight in the neurosciences intensive care unit, where he remained clinically and neurologically stable. Warfarin was restarted on day 2. The patient was ultimately transferred to the stroke neurology service, followed by acute inpatient rehabilitation. At the time of discharge, the patient had persistent left facial droop, left arm paresis with minimal activation, and left hip flexion antigravity without distal activation.
DISCUSSION
In this case report, we present a perioperative ischemic stroke 4 days following an uncomplicated ACDF in a patient with multiple risk factors, including bilateral carotid stenosis, resulting in arterial thrombosis and ischemia ipsilateral to the surgical approach. This is the first case of such a complication occurring beyond the immediate perioperative period, and only the fifth reported cerebral infarct attributable to any etiology after ACDF without corpectomy.[
The patient history demonstrated many risk factors for perioperative stroke of various etiologies, including hypertension, male gender, new onset atrial fibrillation, and history of unprovoked PE requiring anticoagulation. In addition, his warfarin was stopped perioperatively, and during the early postoperative period, he developed dehydration due to systemic illness. We hypothesize that, in this patient with significant underlying atherosclerotic disease, surgical retraction sufficiently disturbed the carotid plaque to create turbulent flow and local hemostasis. In the setting of acute dehydration, compounded by an untreated hypercoagulable state, flow through the carotid artery was critically diminished, precipitating thrombosis and right hemispheric watershed infarct.
Although it is difficult to say with certainty that surgical retraction contributed to this complication, we find the circumstantial evidence compelling. The proposed mechanism finds further support from physiologic studies suggesting that prolonged retraction of an atherosclerotic carotid artery may induce flow-limiting stenosis or precipitate arterial embolic events, via disruption or dislodgement of the plaque by the retractor system.[
From the unique clinical history, a number of alternative mechanisms can be imagined, invoking the patient's history of new atrial fibriallation and previous unprovoked PE. Cessation of anticoagulation in nonvalvular atrial fibrillation is a rare, but not an impossible cause of perioperative stroke, with an estimated incidence of 2 events in 10000 procedures.[
Similarly, in the setting of a potential iatrogenic carotid injury, arterial dissection precipitating thrombosis warrants consideration although our literature review found this to be an even rarer complication of ACDF than carotid thrombosis.[
In retrospect, some consideration is due to whether the elective procedure at the heart of the present case was warranted, or if the preoperative risk factors for thrombosis and other related complications may have outweighed the potential benefit. With knowledge of the outcome in mind, one can assemble a compelling list of arguments in opposition to the intervention, or at minimum, a more thorough preoperative assessment: a history of coagulopathy and clinically significant thrombotic events requiring anticoagulation, substantial atherosclerotic burden, and other related medical comorbidities. In parallel, our review of the literature affirms the scarcity of thrombotic complications attributable to arterial injury after ACDF; taken together with the high incidence of asymptomatic carotid stenosis—up to 3.1% in the general population—and the frequency with which ACDF is performed, it is difficult to compellingly argue against neurosurgical intervention in patients with symptomatic radiculopathy or myelopathy and vascular risk factors alone. Taking these considerations in concert, a more extensive preoperative work-up may provide the optimal balance in high risk patients in need of symptomatic relief.
Central to our recommendations is an individually-tailored patient care plan; however, certain key elements should be weighed in each assessment. These may include consultation with Medicine or Cardiology to better define preoperative risk factors, or carotid artery imaging in appropriately selected individuals.
The question of an ideal surgical approach in the present case is impossible to answer without adequate preoperative vascular imaging; however, based on the postoperative outcome, we can offer some limited insight regarding the viability of either a contralateral anterior or a posterior approach. Postoperative vascular imaging suggests that the patient's severe carotid disease was in fact bilateral, even though potentially less pronounced on the left, highlighting one potentially safer alternative surgical corridor [Figures
CONCLUSION
The implications of this case are challenging to interpret, particularly in the light of the limited evidence regarding carotid injury following ACDF. On the one hand, universal screening for carotid disease with preoperative imaging would be highly cost-ineffective, particularly in an exceedingly low-incidence entity. However, the present case and related reports highlight the impoverished knowledge base surrounding cerebral ischemia as a potential complication of ACDF, inviting speculation that the true incidence may be underestimated by current reports—particularly in the delayed setting.
With this possibility in mind, we recommend a focused review of the medical history for atherosclerosis risk factors in all patients prior to ACDF, with a low threshold for thorough preoperative assessment in patients with significant vascular risk factors and consideration for alternative surgical approaches. In cases with indeterminate histories, physical examination with carotid auscultation may provide an essential data point informing the decision to image, although the low cost and risk of carotid ultrasound should bias toward imaging in unclear circumstances. Similarly, a role may be defined for postoperative carotid imaging to confirm vessel patency and laminar flow among patients with known atherosclerotic burden. In appropriately selected patients, this could open the door to intervene before complications arise.
Finally, we believe that our case argues compellingly in favor of early reinitiation of anticoagulation or antiplatelet therapy, which in the case of an elective ACDF that was not complicated by significant intraoperative hemorrhage may be started within 48 hours of surgery, potentially within the first 12 hours following the operation in patients at highest risk of thrombotic complications. At present, clinical evidence regarding resumption of postoperative anticoagulation is heterogeneous, and the lack of specific information on post-ACDF populations makes it challenging to generalize the limited evidence that does exist, in particular given the rarity of the complication in question.[
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Chin KR, Seale J, Butron V, Cumming V. Postoperative cervical haematoma complicated by ipsilateral carotid thrombosis and aphasia after anterior cervical fusion: A case report. Case Rep Med 2013. 2013. p.
2. Drummond JC, Englander RN, Gallo CJ. Cerebral ischemia as an apparent complication of anterior cervical discectomy in a patient with an incomplete circle of Willis. Anesth Analg. 2006. 102: 896-9
3. Guha D, Coyne S, Macdonald RL. Timing of the resumption of antithrombotic agents following surgical evacuation of chronic subdural hematomas: A retrospective cohort study. J Neurosurg. 2016. 124: 750-9
4. Halbach VV, Higashida RT, Dowd CF, Fraser KW, Smith TP, Teitelbaum GP. Endovascular treatment of vertebral artery dissections and pseudoaneurysms. J Neurosurg. 1993. 79: 183-91
5. Hirsh J, Dalen J, Anderson DR, Poller L, Bussey H, Ansell J. Oral anticoagulants: Mechanism of action, clinical effectiveness, and optimal therapeutic range. Chest. 2001. 119: 8S-21S
6. Kearon C, Hirsh J. Management of anticoagulation before and after elective surgery. N Engl J Med. 1997. 336: 1506-11
7. Loret JE, Francois P, Papagiannaki C, Cottier JP, Terrier LM, Zemmoura I. Internal carotid artery dissection after anterior cervical disc replacement:First case report and literature review of vascular complications of the approach. Eur J Orthop Surg Traumatol. 2013. 23: 107-10
8. Pollard ME, Little PW. Changes in carotid artery blood flow during anterior cervical spine surgery. Spine. 2002. 27: 152-5
9. Radhakrishnan M, Bansal S, Srihari GS, Sampath S, Rao GS. Perioperative stroke following anterior cervical discectomy. Br J Neurosurg. 2010. 24: 592-4
10. Uribe J, Moza K, Jimenez O, Green B, Levi AD. Delayed postoperative spinal epidural hematomas. Spine J. 2003. 3: 125-9
11. Yeh YC, Sun WZ, Lin CP, Hui CK, Huang IR, Lee TS. Prolonged retraction on the normal common carotid artery induced lethal stroke after cervical spine surgery. Spine. 2004. 29: E431-4