- Department of Neurosurgery, Cedars-Sinai Medical Center, Los Angeles, California
- School of Medicine, Stony Brook University, New York, USA
- Department of Neurology, Cedars-Sinai Medical Center, Los Angeles, California
Correspondence Address:
Doniel Drazin
Department of Neurology, Cedars-Sinai Medical Center, Los Angeles, California
DOI:10.4103/2152-7806.200574
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Doniel Drazin, Ari Kappel, Stefan Withrow, Tiffany Perry, Ray Chu, Surasak Phuphanich. Post-irradiation lumbosacral radiculopathy associated with multiple cavernous malformations of the cauda equina: Case report and review of the literature. 20-Feb-2017;8:26
How to cite this URL: Doniel Drazin, Ari Kappel, Stefan Withrow, Tiffany Perry, Ray Chu, Surasak Phuphanich. Post-irradiation lumbosacral radiculopathy associated with multiple cavernous malformations of the cauda equina: Case report and review of the literature. 20-Feb-2017;8:26. Available from: http://surgicalneurologyint.com/surgicalint_articles/post%e2%80%91irradiation-lumbosacral-radiculopathy-associated-with-multiple-cavernous-malformations-of-the-cauda-equina-case-report-and-review-of-the-literature/
Abstract
Background:Multiple radiation-induced cavernous malformations of the cauda equina are extremely rare. A review of the literature suggested that the post-irradiation lumbosacral radiculopathy in our patient was most likely associated with a diagnosis of multiple radiation-induced cavernous malformations of the cauda equina.
Case Description:A 76-year-old man with a remote history of abdominal radiation therapy presented with a 6-month history of progressively worsening right foot drop and balance impairment. Magnetic resonance imaging (MRI) revealed multiple enhancing areas of the cauda equina concerning for carcinomatous meningitis, however, cerebrospinal fluid (CSF) analysis was unrevealing. Intraoperative findings were consistent with multiple radiation-induced cavernous malformations of the cauda equina.
Conclusions:Multiple radiation-induced cavernous malformations of the cauda equina may mimic carcinomatous or infectious meningitis. Clinicians should be suspicious of this diagnosis when CSF and MRI findings are inconsistent with metastatic disease or infectious meningitis in patients who present with radiculopathy and a history of radiation therapy.
Keywords: Cauda equina, cavernous malformation, radiculopathy, radiotherapy, spine
INTRODUCTION
The syndrome of post-irradiation flaccid paralysis was first reported by Greenfield and Stark in 1948.[
CASE REPORT
A 76-year-old male was referred in 2014 with a 6-month history of progressively worsening right foot drop and balance impairment. He had a previous history of abdominal sarcoma treated with surgery, radiation, and chemotherapy in 1980. He had no neurological symptoms at that time.
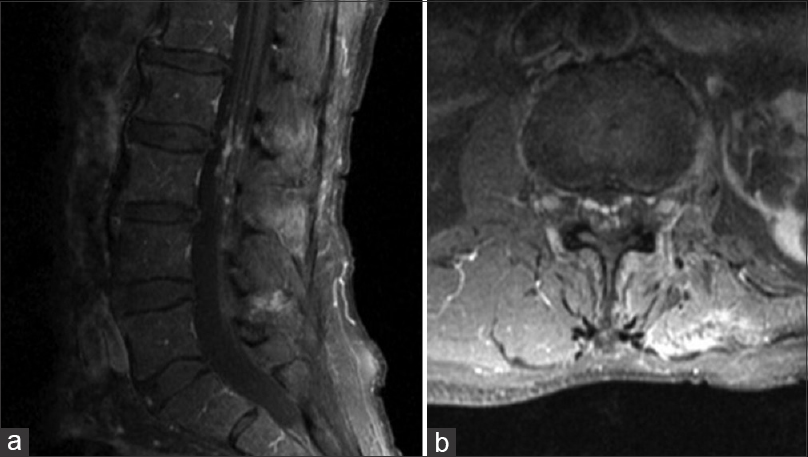
Neurologic examination was normal except for 2/5 strength in the right tibialis anterior and 1/5 strength in the right extensor hallucis longus. Light touch and vibration sensations were intact. Straight leg raise test was negative, and there were no pathological reflexes. The initial MRI showed numerous small (2–3 mm), contrast-enhancing intrathecal lesions involving the cauda equina nerve roots at the levels of the L2 and L3 vertebrae [
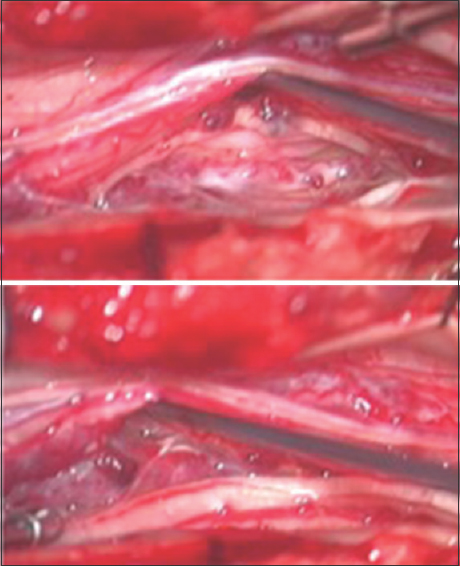
Three months after initial evaluation, the MRI was repeated and appeared stable. The patient underwent L2-L3 laminoplasty with intradural exploration for suspected leptomeningeal carcinomatosis. Intraoperative ultrasound revealed multiple hyperdensities along the cauda equina in the exposed field. The dura and arachnoid were opened under magnification, and the subarachnoid space and cauda equina were examined. Multiple small, mulberry-like nodularlesions associated with dilated vessels were discovered to be intimately involved with the cauda equina [
Figure 2
Intraoperative photograph demonstrating multiple small mulberry-like vascular malformations intimately involved with the nerve roots of the cauda equina. These vascular malformations are associated with dilated vessels, and most likely represent multiple radiation-induced cavernous malformations
The patient was conservatively managed with bedrest, opiates and muscle relaxants and was discharged home with physical and occupational therapy. Spinal angiogram performed at three months and at two years post-operatively showed no abnormal findings. An aortogram was also performed to ensure that no other feeding vessels were identified because of the concern that his prior radiation could have induced stenosis of the vascular supply. This study was also negative. Postoperative MRI was repeated at 6 months, 9 months, and 2 years with stable appearance of the vascular malformations. His current functional status is that he still has the right foot drop and some mild distal weakness on his left.
DISCUSSION
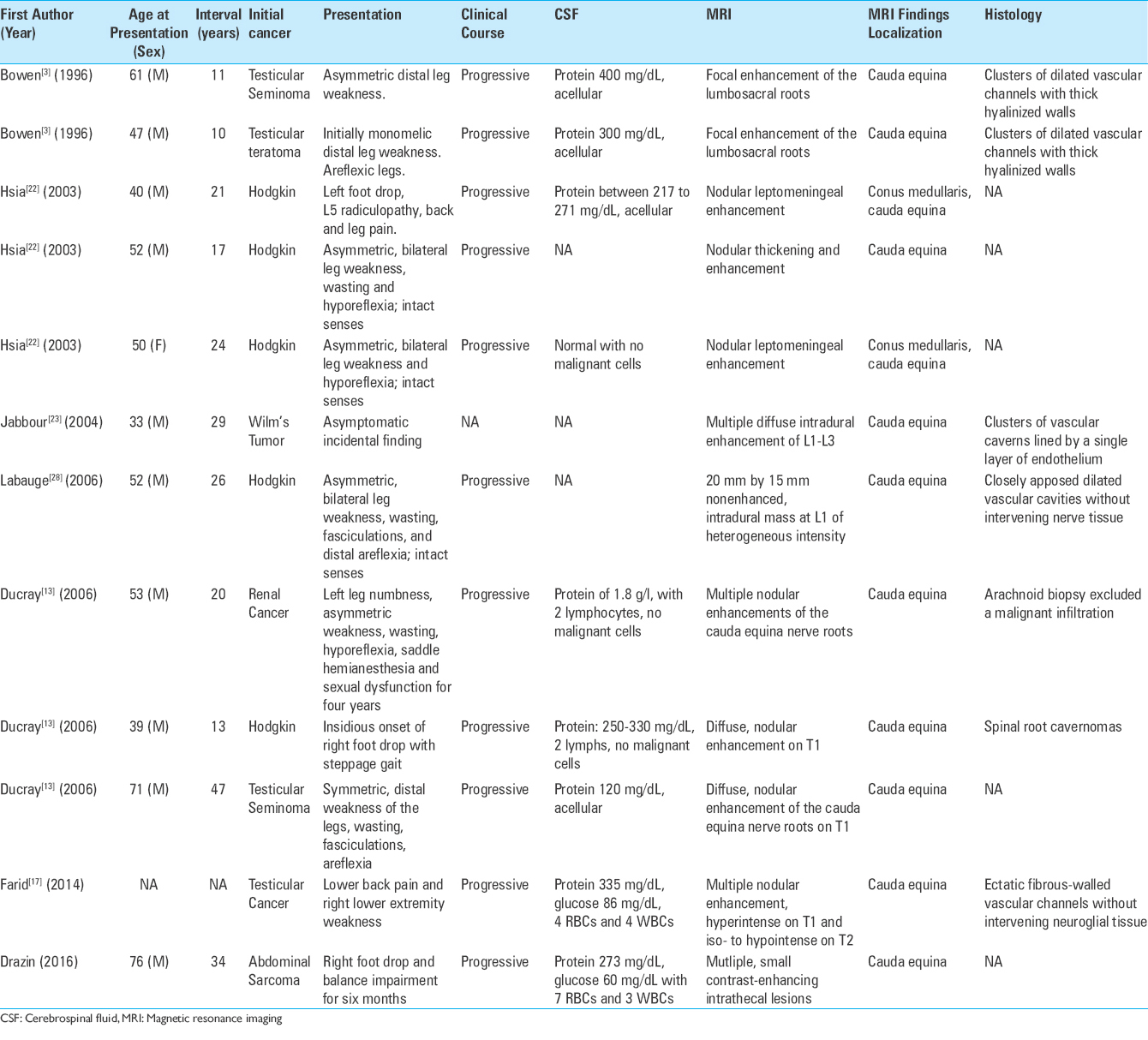
Our patient developed progressive flaccid right foot weakness 34 years after radiation therapy for abdominal sarcoma. Radiological data showed multiple contrast enhancing, angiographically occult nodules along the cauda equina. The absence of malignant cells in serial CSF studies made a diagnosis of leptomeningeal carcinomatosis less likely. On intraoperative exploration, the appearance of multiple mulberry-like vascular lesions associated with dilated vessels was consistent with multiple vascular malformations of the cauda equina. Because of the risk of permanent damage to the nerve roots of the cauda equina, the lesions were not biopsied and a definitive diagnosis could not be made. However, a review of the literature suggests that the diagnosis of multiple radiation-induced spinal cavernous malformations is the most likely diagnosis.
Intradural spinal cavernous malformations are uncommon lesions that account for only 3–13% of all space occupying spinal lesions.[
Histologically, cavernous malformations consist of dilated, thin-walled, and compact endothelial-lined channels, which are devoid of intervening neurological tissue.[
The finding of lower motor neuron signs and multiple nodular enhancements of the cauda equina nerve roots in a patient with a history of malignancy and previous radiation therapy presents a diagnostic challenge.[
In 1996, Bowen et al.[
In 2006, Ducray et al.[
The development of progressive radiculopathy 34 years after lumbar radiation exposure in our patient is consistent with prior cases of post-irradiation lumbosacral radiculopathy with multiple spinal cavernous malformations.[
CONCLUSION
Multiple radiation-induced cavernous malformations of the cauda equina may mimic carcinomatous or infectious meningitis. Cavernous malformations of the cauda equina are uncommon lesions, but are the most likely vascular malformation in the setting of post-irradiation radiculopathy. Clinicians should be suspicious of this diagnosis when CSF and MRI findings are inconsistent with metastatic disease or infectious meningitis in patients who present with lumbosacral radiculopathy and a history of radiation therapy. Preoperative diagnosis may prevent unnecessary biopsy and/or treatment.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Auffray-Calvier E, Desal HA, Freund P, Laplaud D, Mathon G, De Kersaint-Gilly A. Capillary telangiectasis, angiographically occult vascular malformations. MRI symptomatology apropos of 7 cases. J Neuroradiol. 1999. 26: 257-61
2. Berlit P, Schwechheimer K. Neuropathological findings in radiation myelopathy of the lumbosacral cord. Eur Neurol. 1987. 27: 29-34
3. Bowen J, Gregory R, Squier M, Donaghy M. The post-irradiation lower motor neuron syndrome neuronopathy or radiculopathy?. Brain. 1996. 119: 1429-39
4. Bruni P, Massari A, Greco R, Hernandez R, Oddi G, Chiappetta F. Subarachnoid hemorrhage from cavernous angioma of the cauda equina: Case report. Surg Neurol. 1994. 41: 226-9
5. Caroli E, Acqui M, Trasimeni G, Di Stefano D, Ferrante L. A case of intraroot cauda equina cavernous angioma: Clinical considerations. Spinal Cord. 2007. 45: 318-21
6. Cecchi PC, Rizzo P, Faccioli F, Bontempini L, Schwarz A, Bricolo A. Intraneural cavernous malformation of the cauda equina. J Clin Neurosci. 2007. 14: 984-6
7. Cervoni L, Celli P, Gagliardi FM. Cavernous angioma of the cauda equina: Report of two cases and review of the literature. Neurosurg Rev. 1995. 18: 281-3
8. Chun SW, Kim SJ, Lee TH, Koo HS. Intra-root cavernous angioma of the cauda equina: A case report and review of the literature. J Korean Neurosurg Soc. 2010. 47: 291-4
9. Ciricillo SF, Cogen PH, Edwards MS. Pediatric cryptic vascular malformations: Presentation, diagnosis and treatment. Pediatr Neurosurg. 1994. 20: 137-47
10. Clatterbuck RE, Elmaci I, Rigamonti D. The juxtaposition of a capillary telangiectasia, cavernous malformation, and developmental venous anomaly in the brainstem of a single patient: Case report. Neurosurgery. 2001. 49: 1246-50
11. Cohen HC, Tucker WS, Humphreys RP, Perrin RJ. Angiographically cryptic histologically verified cerebrovascular malformations. Neurosurgery. 1982. 10: 704-14
12. Del Curling O, Kelly DL, Elster AD, Craven TE. An analysis of the natural history of cavernous angiomas. J Neurosurg. 1991. 75: 702-8
13. Ducray F, Guillevin R, Psimaras D, Sanson M, Mokhtari K, Delanian S. Postradiation lumbosacral radiculopathy with spinal root cavernomas mimicking carcinomatous meningitis. Neurooncology. 2008. 10: 1035-9
14. Duke BJ, Levy AS, Lillehei KO. Cavernous angiomas of the cauda equina: Case report and review of the literature. Surg Neurol. 1998. 50: 442-5
15. Ebeling JD, Tranmer BI, Davis KA, Kindt GW, DeMasters BK. Thrombosed arteriovenous malformations: A type of occult vascular malformation. Magnetic resonance imaging and histopathological correlations. Neurosurgery. 1988. 23: 605-10
16. Falavigna A, Righesso Neto O, dos Santos JA, Ferraz FA. Cavernous angioma of the cauda equina: Case report. Arq Neuropsiquiatr. 2004. 62: 531-4
17. Farid N, Zyroff J, Uchiyama CM, Thorson PK, Imbesi SG. Radiation-induced cavernous malformations of the cauda equina mimicking carcinomatous or infectious meningitis. A case report. J Neuroimaging. 2014. 24: 92-4
18. Gaensler EH, Dillon WP, Edwards MS, Larson DA, Rosenau W, Wilson CB. Radiation-induced telangiectasia in the brain simulates cryptic vascular malformations at MR imaging. Radiology. 1994. 193: 629-36
19. Greenfield MM, Stark FM. Post-irradiation neuropathy. Am J Roentgenol Radium Ther. 1948. 60: 617-22
20. Heckl S, Aschoff A, Kunze S. Radiation-induced cavernous hemangiomas of the brain: A late effect predominantly in children. Cancer. 2002. 94: 3285-91
21. Hirsch J, Pradat P, David M. Angiome caverneux de la queue de cheval. Neurochirurgie. 1965. 11: 323-7
22. Hsia AW, Katz JS, Hancock SL, Peterson K. Post-irradiation polyradiculopathy mimics leptomeningeal tumor on MRI. Neurology. 2003. 60: 1694-6
23. Jabbour P, Gault J, Murk SE, Awad IA. Multiple spinal cavernous malformations with atypical phenotype after prior irradiation: Case report. Neurosurgery. 2004. 55: 1431-
24. Jain R, Robertson PL, Gandhi D, Gujar SK, Muraszko KM, Gebarski S. Radiation-induced cavernomas of the brain. AJNR Am J Neuroradiol. 2005. 26: 1158-62
25. Jellinger K, Pia HW, Djindjian R.editors. Pathology of Spinal Vascular Malformations and Vascular Tumors. Spinal Angiomas: Advances in Diagnosis and Therapy. Berlin, Heidelberg: Springer Berlin Heidelberg; 1978. p. 18-44
26. Koike S, Aida N, Hata M, Fujita K, Ozawa Y, Inoue T. Asymptomatic radiation-induced telangiectasia in children after cranial irradiation: Frequency, latency, and dose relation. Radiology. 2004. 230: 93-9
27. Kristensen O, Melgard B, Schiodt AV. Radiation myelopathy of the lumbo-sacral spinal cord. Acta Neurol Scand. 1977. 56: 217-22
28. Labauge P, Lefloch A, Chapon F, Castelnovo G, Maubon A, Rigau V. Postirradiation spinal root cavernoma. Eur Neurol. 2006. 56: 256-7
29. Lamy C, Mas JL, Varet B, Ziegler M, de Recondo J. Postradiation lower motor neuron syndrome presenting as monomelic amyotrophy. J Neurol Neurosurg Psychiatry. 1991. 54: 648-9
30. Larson JJ, Ball WS, Bove KE, Crone KR, Tew JM. Formation of intracerebral cavernous malformations after radiation treatment for central nervous system neoplasia in children. J Neurosurg. 1998. 88: 51-6
31. Lobato RD, Perez C, Rivas JJ, Cordobes F. Clinical, radiological, and pathological spectrum of angiographically occult intracranial vascular malformations. Analysis of 21 cases and review of the literature. J Neurosurg. 1988. 68: 518-31
32. Maeder P, Gudinchet F, Meuli R, de Tribolet N. Development of a cavernous malformation of the brain. AJNR Am J Neuroradiol. 1998. 19: 1141-3
33. Maier JG, Perry RH, Saylor W, Sulak MH. Radiation myelitis of the dorsolumbar spinal cord. Radiology. 1969. 93: 153-60
34. Makino K, Takamura H, Gotoh S, Andoh M. Cauda equina cavernous hemangioma associated with hydrocephalus-case report. No to shinkei. 1995. 47: 783-7
35. Miyake S, Uchihashi Y, Takaishi Y, Sakagami Y, Kohmura E. Multiple cavernous angiomas of the cauda equina. Case report. Neurol Med Chir. 2007. 47: 178-81
36. Moreno R, Romero J, Serrano V, Madrid A, Jarrín S, Casado J. Cavernoma intradural extramedular de cola de caballo. Rev Neurol. 1995. 23: 1228-30
37. Nie QB, Chen Z, Jian FZ, Wu H, Ling F. Cavernous angioma of the cauda equina: A case report and systematic review of the literature. J Int Med Res. 2012. 40: 2001-8
38. Olivero WC, Deshmukh P, Gujrati M. Radiation-induced cavernous angioma mimicking metastatic disease. Br J Neurosurg. 2000. 14: 575-8
39. Pagni CA, Canavero S, Forni M. Report of a cavernoma of the cauda equina and review of the literature. Surg Neurol. 1990. 33: 124-31
40. Pansini A. F. LR. Raro case di angiocavernoma della cauda. Men Soc Tos Um Chir. 1966. 27: 679-6
41. Ramos F, de Toffol B, Aesch B, Jan M. Hydrocephalus and cavernoma of the cauda equina. Neurosurgery. 1990. 27: 139-42
42. Reagan TJ, Thomas JE, Colby MY. Chronic progressive radiation myelopathy. Its clinical aspects and differential diagnosis. JAMA. 1968. 203: 106-10
43. Rigamonti D, Drayer BP, Johnson PC, Hadley MN, Zabramski J, Spetzler RF. The MRI appearance of cavernous malformations (angiomas). J Neurosurg. 1987. 67: 518-24
44. Rigamonti D, Hadley MN, Drayer BP, Johnson PC, Hoenig-Rigamonti K, Knight JT. Cerebral cavernous malformations. Incidence and familial occurrence. N Engl J Med. 1988. 319: 343-7
45. Russell DS, Rubinstein LJ.editorsPathology of Tumors of the Nervous System. Baltimore: Williams and Wilkins; 1989. p. 727-36
46. Sadowsky CH, Sachs E, Ochoa J. Postradiation motor neuron syndrome. Arch Neurol. 1976. 33: 786-7
47. Tomlinson FH, Houser OW, Scheithauer BW, Sundt TM, Okazaki H, Parisi JE. Angiographically occult vascular malformations: A correlative study of features on magnetic resonance imaging and histological examination. Neurosurgery. 1994. 34: 792-800
48. Ueda S, Saito A, Inomori S, Kim I. Cavernous angioma of the cauda equina producing subarachnoid hemorrhage. Case report. J Neurosurg. 1987. 66: 134-6
49. Valk PE, Dillon WP. Radiation injury of the brain. AJNR Am J Neuroradiol. 1991. 12: 45-62
50. Zabramski JM, Wascher TM, Spetzler RF, Johnson B, Golfinos J, Drayer BP. The natural history of familial cavernous malformations: Results of an ongoing study. J Neurosurg. 1994. 80: 422-32






Catriona Gatland
Posted September 6, 2018, 10:48 pm
I have post radiation lumbosacral radiculopathy 28 years after radiotherapy and chemo for Hodgkins disease. As it is a progressive problem, I am keen to learn more about it. I am having a lot of problems with mobility now and wonder if I can expect further after effects. Because this is a rare complication, I cannot talk to anyone knowledgeable in this condition. Thank you, in anticipation
jim
Posted September 19, 2018, 1:21 pm
Unfortunately, we are not able to offer any medical advice. We wish you the best of luck.
SNI