- Department of Neurosurgery, Baylor College of Medicine/Texas Children's Hospital, Houston, TX 77030, USA
Correspondence Address:
Sandi Lam
Department of Neurosurgery, Baylor College of Medicine/Texas Children's Hospital, Houston, TX 77030, USA
DOI:10.4103/2152-7806.178572
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Gadgil N, Hansen D, Barry J, Chang R, Lam S. Posterior fossa syndrome in children following tumor resection: Knowledge update. Surg Neurol Int 11-Mar-2016;7:
How to cite this URL: Gadgil N, Hansen D, Barry J, Chang R, Lam S. Posterior fossa syndrome in children following tumor resection: Knowledge update. Surg Neurol Int 11-Mar-2016;7:. Available from: http://surgicalneurologyint.com/surgicalint_articles/posterior-fossa-syndrome-in-children-following-tumor-resection-knowledge-update/
Abstract
Keywords: Cerebellar mutism, mutism, pediatric, posterior fossa syndrome, posterior fossa tumor
ILLUSTRATIVE CASES
Case 1
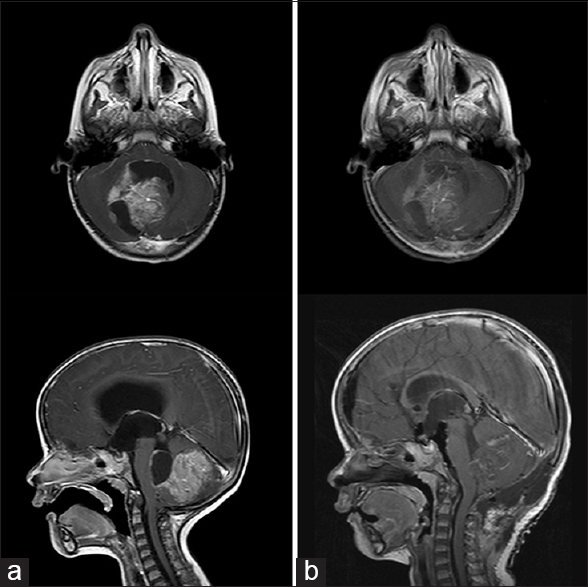
A 2-year-old female presented with a 2-month history of recalcitrant vomiting followed by ataxia and lethargy. Computed tomography (CT) scan of the head showed a large solid and cystic mass in the cerebellar vermis with severe hydrocephalus. Magnetic resonance imaging (MRI) demonstrated a 6.0 cm × 4.4 cm × 4.2 cm uniformly enhancing mass concerning for medulloblastoma with no evidence of spinal metastasis. She underwent external ventricular drain (EVD) placement and uneventful posterior fossa craniotomy with gross total resection of the lesion [
Figure 1
(a) Axial and sagittal T1 postcontrast magnetic resonance images. Large heterogeneous tumor, dorsal to the brainstem and occupying much of the posterior fossa with resulting obstructive hydrocephalus. (b) Postresection images in similar planes showing gross total resection of tumor and resulting decompression of brain stem and ventricular system
Case 2
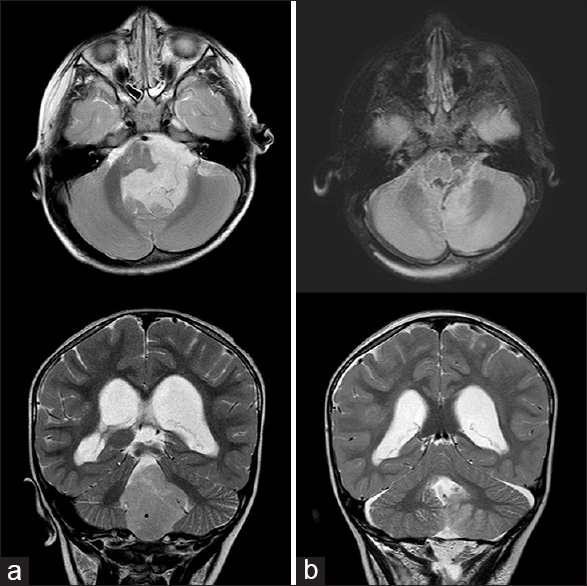
A 2-year-old boy presented with a 3-week history of progressive headache and daily vomiting. A CT scan revealed a solid mass in the fourth ventricle with moderate obstructive hydrocephalus. Presurgical MRI confirmed a 6.1 cm × 4.9 cm × 4.3 cm mass centered in the fourth ventricle and extending out the foramen of Luschka, consistent with an ependymoma. EVD placement and resection of the tumor were carried out in the same setting [
Figure 2
(a) Fluid-attenuated inversion recovery axial and T2 coronal magnetic resonance images. Large nonenhancing tumor, wrapping ventral to the brainstem and occupying the posterior fossa with resultant hydrocephalus. (b) Same sequence postresection images showing gross total resection of tumor, with mild reduction in ventricular caliber
INTRODUCTION
Cerebellar mutism syndrome (CMS) refers to constellation of symptoms noted most commonly following surgery for posterior fossa tumors in the pediatric population. Mutism is a prominent, though not exclusive, characteristic of the syndrome and was first described in 1985 by Rekate et al.[
CLINICAL PRESENTATION, EPIDEMIOLOGY, AND NATURAL HISTORY
CMS occurs in 8–24% of children following resection of posterior fossa masses.[
The duration of mutism varies widely, with an average of approximately 8 weeks, but a range of 4 days to 5 months.[
ETIOLOGY AND PATHOPHYSIOLOGY
Despite collective efforts to describe the pathophysiological mechanism of CMS, the answer remains elusive. Several predisposing risk factors have been observed. Tumor pathology has proven the most predictive, with medulloblastoma patients experiencing a two- to three-fold increased chance of developing CMS as compared to other posterior fossa tumors.[
Although there is no clear consensus in the literature, many associate damage to the dentato-thalamo-cortical pathway with CMS.[
Damage to the vermis may also be important in the development of CMS. The vermis is implicated in speech initiation; while splitting of the inferior third of the vermis is not thought to increase CMS, damage to the superior vermis is considered to be a higher risk.[
Bilateral dentate nuclei damage is also theorized to result in CMS, a theory backed by early work demonstrating mutism following stereotactic lysis of the dentate nuclei for dyskinesia.[
Direct damage to cerebellar neuronal pathways fails to explain why many patients are initially intact postoperatively and develop deficits after a few days, a finding that has led to speculation about other mechanisms. The onset of mutism coincides with the peak timing of postoperative edema;[
PREVENTION AND TREATMENT
To date, no specific treatment has been found for CMS other than supportive care. At our institution, many patients with this syndrome have been referred for intensive inpatient rehabilitation. Patients may require gastrostomy tube placement and intensive speech, physical, and occupational therapy. Although our outcomes have been positive, recovery is gradual and may remain incomplete. For patients with primarily dysarthric speech disorders, exercises focused on coordination of sensorimotor integration should be emphasized. Other patients may have an apraxic language disorder, in which procedural memory and recognition of sensory stimuli is defective; this manifests in slow, monotone speech. Emphasis for these patients should be placed on the awareness of visual and auditory stimuli and planning of sound sequences.[
Several groups have reported single patient trials of pharmaceutical therapies for CMS, including steroids, fluoxetine, bromocriptine, or zolpidem.[
Our lack of understanding of the precise pathophysiologic mechanism of CMS makes it difficult to accurately forecast which patients will develop the syndrome. While several predictive factors have been found, none can be used with certainty. Therefore, our hope at this time is prevention of damage from aggressive tumor resection.[
CONCLUSION
CMS is a common but devastating complication of posterior fossa surgery in children. While the mutism itself is often transient, permanent sequelae are common. The precise pathophysiology of this disease remains unknown, and treatment focuses on supportive care symptoms. At Texas Children's Hospital, multidisciplinary evaluation and treatment are integral to brain tumor care. Physical medicine and rehabilitation, neurology, ophthalmology, and neuro-oncology teams evaluate and follow patients; those with continuing therapy needs transition to intensive inpatient rehabilitation after surgery. Further investigation as to the underlying mechanism of CMS likely holds the promise of prevention and treatment of this syndrome.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Adachi J, Nishikawa R, Hirose T, Matsutani M. Mixed neuronal-glial tumor of the fourth ventricle and successful treatment of postoperative mutism with bromocriptine: Case report. Surg Neurol. 2005. 63: 375-9
2. Aguiar PH, Plese JP, Ciquini O, Marino R. Transient mutism following a posterior fossa approach to cerebellar tumors in children: A critical review of the literature. Childs Nerv Syst. 1995. 11: 306-10
3. Akhaddar A, Salami M, El Asri AC, Boucetta M. Treatment of postoperative cerebellar mutism with fluoxetine. Childs Nerv Syst. 2012. 28: 507-8
4. Baillieux H, Weyns F, Paquier P, De Deyn PP, Mariën P. Posterior fossa syndrome after a vermian stroke: A new case and review of the literature. Pediatr Neurosurg. 2007. 43: 386-95
5. Catsman-Berrevoets CE, Van Dongen HR, Mulder PG, Paz y Geuze D, Paquier PF, Lequin MH. Tumour type and size are high risk factors for the syndrome of “cerebellar” mutism and subsequent dysarthria. J Neurol Neurosurg Psychiatry. 1999. 67: 755-7
6. Clerico A, Sordi A, Ragni G, Festa A, Cappelli C, Maini CL. Brief report: Transient mutism following posterior fossa surgery studied by single photon emission computed tomography (SPECT). Med Pediatr Oncol. 2002. 38: 445-8
7. De Smet HJ, Mariën P. Posterior fossa syndrome in an adult patient following surgical evacuation of an intracerebellar haematoma. Cerebellum. 2012. 11: 587-92
8. Doxey D, Bruce D, Sklar F, Swift D, Shapiro K. Posterior fossa syndrome: Identifiable risk factors and irreversible complications. Pediatr Neurosurg. 1999. 31: 131-6
9. Drost G, Verrips A, Thijssen HO, Gabreëls FJM. Cerebellar involvement as a rare complication of pneumococcal meningitis. Neuropediatrics. 2000. 31: 97-9
10. El-Bahy K. Telovelar approach to the fourth ventricle: Operative findings and results in 16 cases. Acta Neurochir (Wien). 2005. 147: 137-42
11. El-Nabbout B, DeLong G.editors. Treatment of cerebellar mutism with fluoxetine: Report on two patients. Annals of Neurology. New York: Wiley-Liss, Div John Wiley & Sons Inc; 2002. p.
12. Ersahin Y, Mutluer S, Cagli S, Duman Y. Cerebellar mutism: Report of seven cases and review of the literature. Neurosurgery. 1996. 38: 60-5
13. Fraioli B, Guidetti B. Effects of stereotactic lesions of the dentate nucleus of the cerebellum in man. Appl Neurophysiol. 1975. 38: 81-90
14. Fujisawa H, Yonaha H, Okumoto K, Uehara H, Ie T, Nagata Y. Mutism after evacuation of acute subdural hematoma of the posterior fossa. Childs Nerv Syst. 2005. 21: 234-6
15. Gelabert-González M, Fernández-Villa J. Mutism after posterior fossa surgery. Review of the literature. Clin Neurol Neurosurg. 2001. 103: 111-4
16. Hermann EJ, Rittierodt M, Krauss JK. Combined transventricular and supracerebellar infratentorial approach preserving the vermis in giant pediatric posterior fossa midline tumors. Neurosurgery. 2008. 63: ONS30-5
17. Levisohn L, Cronin-Golomb A, Schmahmann JD. Neuropsychological consequences of cerebellar tumour resection in children: Cerebellar cognitive affective syndrome in a paediatric population. Brain. 2000. 123: 1041-50
18. McMillan HJ, Keene DL, Matzinger MA, Vassilyadi M, Nzau M, Ventureyra EC. Brainstem compression: A predictor of postoperative cerebellar mutism. Childs Nerv Syst. 2009. 25: 677-81
19. Morris EB, Phillips NS, Laningham FH, Patay Z, Gajjar A, Wallace D. Proximal dentatothalamocortical tract involvement in posterior fossa syndrome. Brain. 2009. 132: 3087-95
20. Mortimer DS. Clinical case study: A 4-year-old boy with posterior fossa syndrome after resection of a medulloblastoma. J Neurosci Nurs. 2011. 43: 225-9
21. Ojemann JG, Partridge SC, Poliakov AV, Niazi TN, Shaw DW, Ishak GE. Diffusion tensor imaging of the superior cerebellar peduncle identifies patients with posterior fossa syndrome. Childs Nerv Syst. 2013. 29: 2071-7
22. Palmer SL, Hassall T, Evankovich K, Mabbott DJ, Bonner M, Deluca C. Neurocognitive outcome 12 months following cerebellar mutism syndrome in pediatric patients with medulloblastoma. Neuro Oncol. 2010. 12: 1311-7
23. Papavasiliou AS, Kotsalis C, Trakadas S. Transient cerebellar mutism in the course of acute cerebellitis. Pediatr Neurol. 2004. 30: 71-4
24. Pollack IF. Posterior fossa syndrome. Int Rev Neurobiol. 1997. 41: 411-32
25. Pollack IF, Polinko P, Albright AL, Towbin R, Fitz C. Mutism and pseudobulbar symptoms after resection of posterior fossa tumors in children: Incidence and pathophysiology. Neurosurgery. 1995. 37: 885-93
26. Puget S, Boddaert N, Viguier D, Kieffer V, Bulteau C, Garnett M. Injuries to inferior vermis and dentate nuclei predict poor neurological and neuropsychological outcome in children with malignant posterior fossa tumors. Cancer. 2009. 115: 1338-47
27. Rekate HL, Grubb RL, Aram DM, Hahn JF, Ratcheson RA. Muteness of cerebellar origin. Arch Neurol. 1985. 42: 697-8
28. Riva D, Giorgi C. The cerebellum contributes to higher functions during development: Evidence from a series of children surgically treated for posterior fossa tumours. Brain. 2000. 123: 1051-61
29. Robertson PL, Muraszko KM, Holmes EJ, Sposto R, Packer RJ, Gajjar A. Incidence and severity of postoperative cerebellar mutism syndrome in children with medulloblastoma: A prospective study by the Children's Oncology Group. J Neurosurg. 2006. 105: 444-51
30. Shyu C, Burke K, Souweidane MM, Dunkel IJ, Gilheeney SW, Gershon T. Novel use of zolpidem in cerebellar mutism syndrome. J Pediatr Hematol Oncol. 2011. 33: 148-9
31. Siffert J, Poussaint TY, Goumnerova LC, Scott RM, LaValley B, Tarbell NJ. Neurological dysfunction associated with postoperative cerebellar mutism. J Neurooncol. 2000. 48: 75-81
32. Steinbok P, Cochrane DD, Perrin R, Price A. Mutism after posterior fossa tumour resection in children: Incomplete recovery on long-term follow-up. Pediatr Neurosurg. 2003. 39: 179-83
33. Tamburrini G, Frassanito P, Chieffo D, Massimi L, Caldarelli M, Di Rocco C. Cerebellar mutism. Childs Nerv Syst. 2015. 31: 1841-51
34. van Baarsen K, Kleinnijenhuis M, Konert T, van Cappellen van Walsum AM, Grotenhuis A. Tractography demonstrates dentate-rubro-thalamic tract disruption in an adult with cerebellar mutism. Cerebellum. 2013. 12: 617-22
35. Van Calenbergh F, Van de Laar A, Plets C, Goffin J, Casaer P. Transient cerebellar mutism after posterior fossa surgery in children. Neurosurgery. 1995. 37: 894-8
36. van Dongen HR, Catsman-Berrevoets CE, van Mourik M. The syndrome of ‘cerebellar’ mutism and subsequent dysarthria. Neurology. 1994. 44: 2040-6
37. Vandeinse D, Hornyak JE. Linguistic and cognitive deficits associated with cerebellar mutism. Pediatr Rehabil. 1997. 1: 41-4
38. Wells EM, Khademian ZP, Walsh KS, Vezina G, Sposto R, Keating RF. Postoperative cerebellar mutism syndrome following treatment of medulloblastoma: Neuroradiographic features and origin. J Neurosurg Pediatr. 2010. 5: 329-34
39. Wells EM, Walsh KS, Khademian ZP, Keating RF, Packer RJ. The cerebellar mutism syndrome and its relation to cerebellar cognitive function and the cerebellar cognitive affective disorder. Dev Disabil Res Rev. 2008. 14: 221-8
40. Zaheer SN, Wood M. Experiences with the telovelar approach to fourth ventricular tumors in children. Pediatr Neurosurg. 2010. 46: 340-3