- Department of Neurosurgery, Stroke Center, Sapporo Teishinkai Hospital, Hokkaido, Japan
Correspondence Address:
Rokuya Tanikawa
Department of Neurosurgery, Stroke Center, Sapporo Teishinkai Hospital, Hokkaido, Japan
DOI:10.4103/2152-7806.196774
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Nakao Ota, Rokuya Tanikawa, Masataka Miyama, Takanori Miyazaki, Yu Kinoshita, Hidetoshi Matsukawa, Takeshi Yanagisawa, Fumihiro Sakakibara, Norihiro Saito, Shiro Miyata, Kosumo Noda, Toshiyuki Tsuboi, Rihei Takeda, Hiroyasu Kamiyana, Sadahisa Tokuda. Radical resection of a craniopharyngioma via the extradural anterior temporal approach with zygomatic arch osteotomy. 26-Dec-2016;7:
How to cite this URL: Nakao Ota, Rokuya Tanikawa, Masataka Miyama, Takanori Miyazaki, Yu Kinoshita, Hidetoshi Matsukawa, Takeshi Yanagisawa, Fumihiro Sakakibara, Norihiro Saito, Shiro Miyata, Kosumo Noda, Toshiyuki Tsuboi, Rihei Takeda, Hiroyasu Kamiyana, Sadahisa Tokuda. Radical resection of a craniopharyngioma via the extradural anterior temporal approach with zygomatic arch osteotomy. 26-Dec-2016;7:. Available from: http://surgicalneurologyint.com/surgicalint_articles/radical-resection-of-a-craniopharyngioma-via-the-extradural-anterior-temporal-approach-with-zygomatic-arch-osteotomy/
Abstract
Background:Though the extradural anterior temporal approach (EDATA) with zygomatic osteotomy is useful, there are only few reports of this approach being used for craniopharyngioma resection. Herein, we report our surgical case series and the technical importance of EDATA for the radical removal of a craniopharyngioma.
Methods:We report 7 cases of craniopharyngiomas treated surgically between April 1999 and October 2015. The surgical approaches, clinical presentation, pre and postoperative radiographic examination results, surgical outcomes, and morbidity were analyzed.
Results:The mean follow-up period was 89.1 months. The surgical approach was EDATA with zygomatic osteotomy in 4, combined interhemispheric translamina terminalis approach (IHTLA) and trans-sylvian anterior temporal approach (ATA) in 2, and IHTLA in 1 patient. Complete tumor resection was achieved in all cases, without any recurrence during the follow-up period. Transient morbidities were oculomotor nerve palsy in 2, and meningitis and hydrocephalus in 1 patient. There was 1 case of permanent morbidity due to hydrocephalus that needed a ventriculoperitoneal shunt, and 1 case of blindness on the operative side. Visual acuity and visual field improved in 4 cases, showed no change in 2 cases, and deteriorated in 1 case. Though the pituitary stalk was preserved in 2 cases, all 7 cases needed total hormone replacement therapy.
Conclusion:EDATA with zygomatic osteotomy ensures sufficient mobility of the internal carotid artery, and provides a good lateral and look up operative view. Hence, it can be used effectively for radical resection of craniopharyngiomas through the opticocarotid space and retrocarotid space.
Keywords: Anterior clinoidectomy, craniopharyngioma, extradural anterior temporal approach, transzygomatic approach
INTRODUCTION
Craniopharynigomas are usually benign tumors. However, their treatment is not easy because they invade vital structures such as the pituitary stalk, hypothalamus, optic nerve and optic tract, and vessels of circles of Willis. Radical resection in the first operation is important because the recurrence rate increases dramatically in case of incomplete tumor resection, and resection becomes more difficult upon successive operations than in the first operation.[
Several surgical approaches have been reported for this tumor, including the trans-sphenoidal,[
MATERIALS AND METHODS
This retrospective study was approved by our institutional review and ethical board. Patients’ demographic data were obtained from their history and medical records. Supplemental information from charts, operative notes, and radiographic reports was also obtained.
Between April 1999 and Oct 2015, 7 patients diagnosed with craniopharyngioma were treated surgically at our institution. These included 1 male and 6 female patients (age, 10–70 years, median age, 32 years).
Radiological evaluation was performed by computed tomography (CT), CT angiography (CTA), and magnetic resonance imaging (MRI) before operation. The tumor was characterized by type (predominantly cystic, solid, or mixed) and location, according to the classification by Yasargil et al.[
Out of the 7 operations, 4 used EDATA with zygomatic osteotomy, 2 were performed using combined interhemispheric translamina terminalis and trans-sylvian anterior temporal approach (ATA), and 1 was performed using the interhemispheric translamina terminalis approach.
Total resection was defined not only by intraoperative findings but also by postoperative radiographic evaluation using CT and MRI, performed immediately after the operation, and MRI performed before discharge, 3 to 6 months after the operation and then, annually. The outpatient clinic also followed the patients for 1 to 3 months.
Surgical intervention
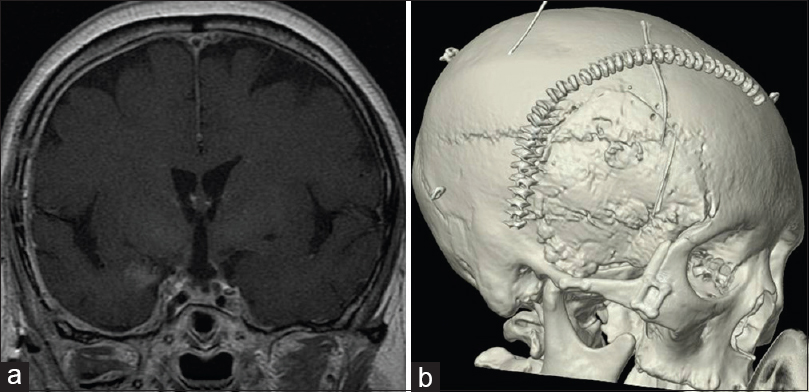
Illustrative case: Case 7, a 70-year-old woman with a solid-type craniopharyngioma [
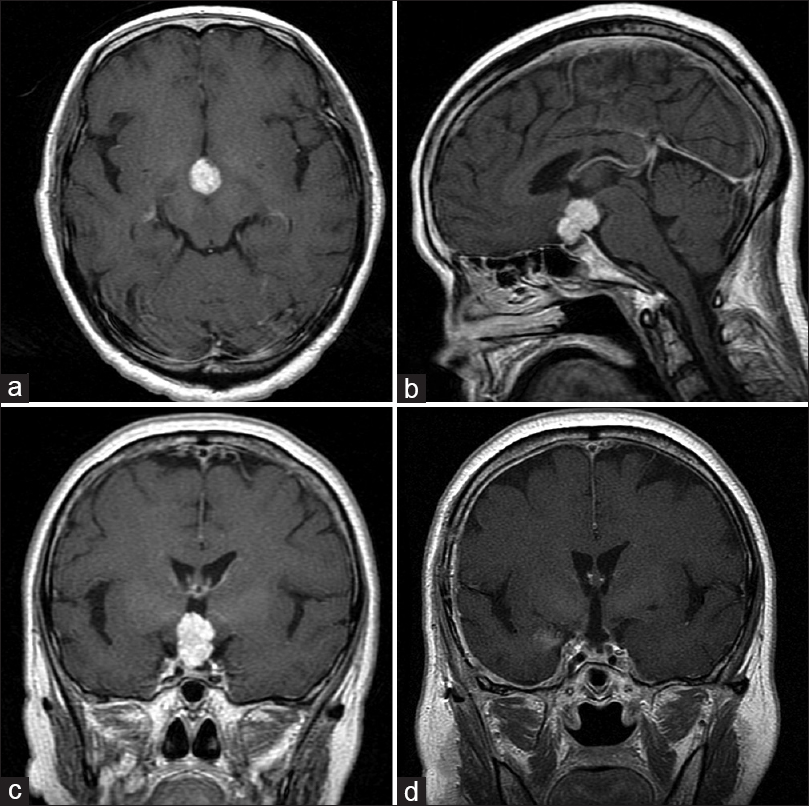
Figure 1
The pre and postoperative magnetic resonance images (MRI) of case 7. (a) Axial image of gadolinium (Gd)-enhanced T1-weighted MRI. Solid well-enhanced tumors exist in the interpeduncular cistern. (b) Saggital image of Gd-enhanced T1-weighted MRI. The tumor exists in the supradiaphragmatic space. (c) Coronal image of Gd-enhanced T1-weighted MRI. (d) Postoperative coronal image of Gd-enhanced T1-weighted MRI. The tumor has been removed completely
Zygomatic osteotomy [ Video 1 ]
Our technique of zygomatic arch osteotomy and anterior clinoidectomy is a modified version of the skull base technique by Dr. Fukushima.[
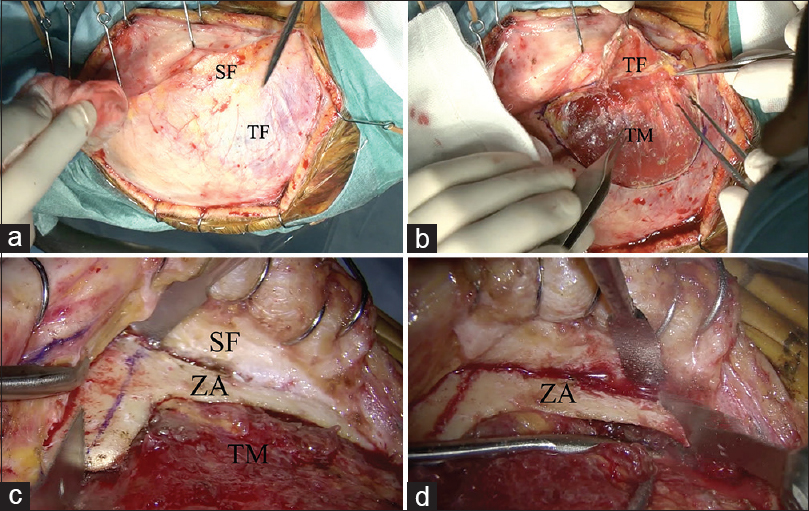
Figure 2
Surgical procedure of zygomatic arch osteotomy. (a) Two-layer skin flap elevation is performed. (b) The superficial fat pad is elevated with the temporal fascia. (c) The temporal muscle is freed from the temporal squama. The zygomatic arch is fully exposed and cut with a sagittal saw. (d) The posterior base of the zygomatic arch is cut with a sagittal saw. “T bone shape” zygomatic arch osteotomy is achieved. SF: superficial fad pad, TF: Temporal fascia, TM: Temporal muscle, ZA: Zygomatic arch
Orbital skeletonization and extradural anterior clinoidectomy [ Video 2 ]
The dura was elevated extradurally and retracted with extradural steel tapered retractors. The orbital roof and the lesser wing of the sphenoid bone were drilled and shaved flat using a 5-mm extra-coarse diamond burr to remove the bony protrusions. To make a “look up” operative field, orbital flattening is essential [Figure
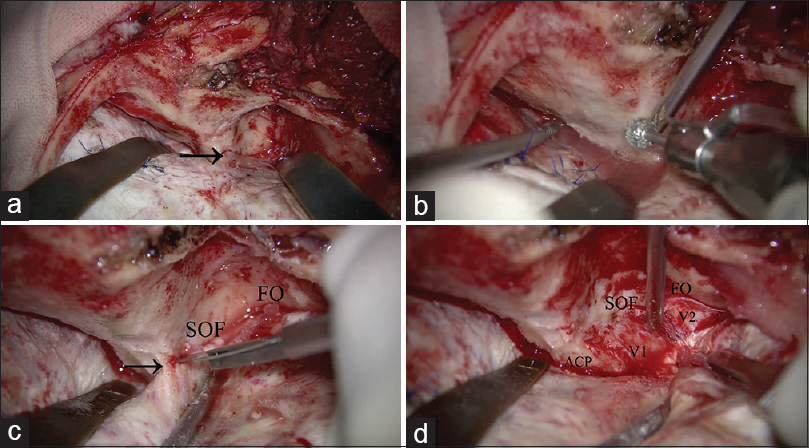
Figure 3
Surgical procedure of anterior clinoidectomy. (a) After zygomatic arch osteotomy, front-temporal craniotomy is performed. The temporal muscle is retracted inferoposteriorly. The lesser wing of the sphenoid bone is partially drilled, and the meningo-orbital band is identified (arrow). (b) Orbital unroofing is performed. (c) The superior orbital fissure and foramen ovale are identified. The meningo-orbital band is cut (arrow). (d) The temporal tip dura is peeled away from the anterior aspect of the cavernous sinus. SOF: Superior orbital fissure, FO: Foramen ovale, ACP: Anterior clinoid process, V1: Ophthalmic nerve, V2: Maxillary nerve
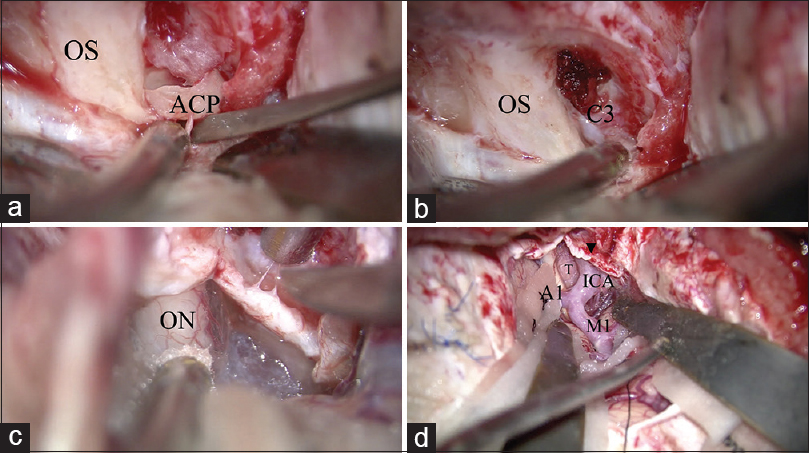
Figure 4
Surgical procedure of anterior clinoidectomy. (a) After drilling the inside of the anterior clinoid process (ACP), the ACP is removed using a small dissector. (b) After removing the ACP, the residual optic strut is drilled. The C3 portion of the internal carotid artery is identified. (c) The optic sheath is opened to decompress the optic nerve. (d) The operative view after anterior clinoidectomy, optic sheath opening, and proximal sylvian fissure opening. The tumor is identified in the opticocarotid space. OS: optic sheath, ACP: Anterior clinoid process, C3: C3 portion of the internal carorid artery, ON: Optic nerve, ICA: Internal caroid artery, M1: M1 portion of the middle cerebral artery, A1: A1 portion of the anterior cerebral artery, T: tumor
The frontotemporal dura was curvilinearly incised along the orbital ridge. The optic sheath was opened to decompress the optic nerve [
Figure 5
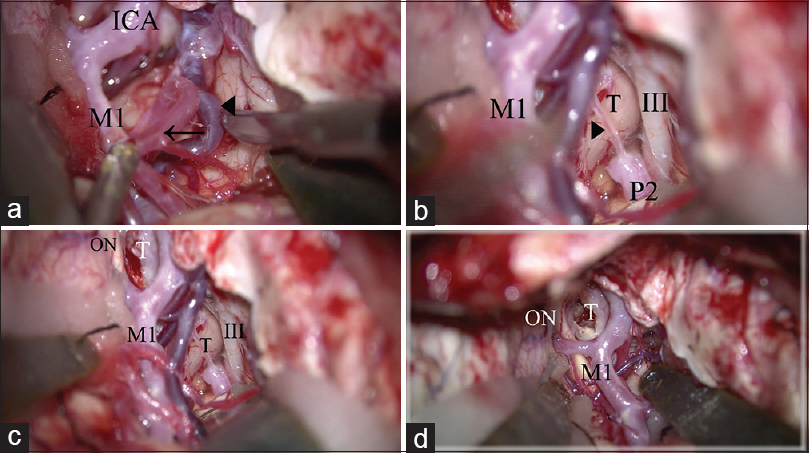
Surgical procedure of extradural anterior temporal approach. (a) The anterior temporal artery (arrow) and deep middle cerebral vein (arrow head) is freed from the temporal lobe to retract the temporal lobe. (b) The arachnoid trabeculae between the oculomotor nerve and the temporal uncus are cut. The posterior communicating artery is identified (arrow head). (c) The temporal lobe is retracted posterolaterally. The retrocarotid space is widely secured. (d) The operative view in the simple trans-sylvian approach before performing the above 2 steps (a, b). The retrocarotid space is narrow and cannot be seen from lateral trajectory compared to c. ICA: Internal carotid artery, M1: M1 portion of the middle cerebral artery, III: Oculomotor nerve, P2: P2 portion of the posterior cerebral artery, T: Tumor, ON: Optic nerve
Craniopharyngioma resection [Videos 3 and 4 ]
To remove the craniopharyngioma, the prechiasmatic space, opticocarotid space, and retrocarotid space should be used [
Figure 6
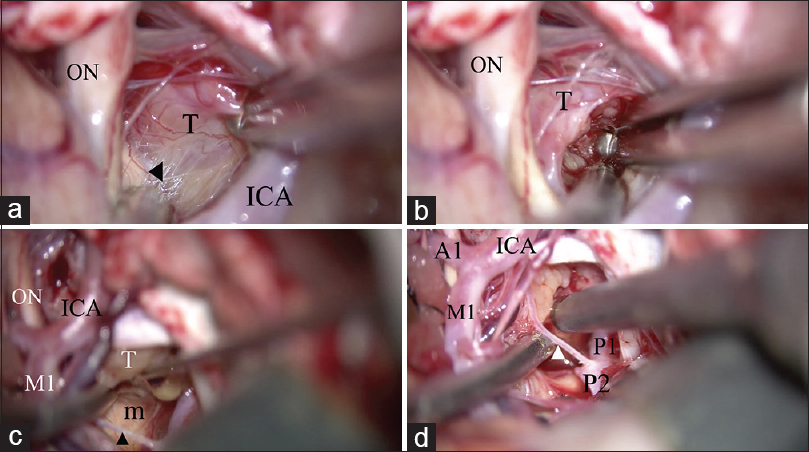
Removal of the craniopharyngioma through both the opticocarotid space and the retrocarotid space. (a) The tumor is dissected from the optic tract (arrow head) via the optico-carotid space. A good “look up” operative view is achieved. (b) Internal decompression of the tumor from the opticocarotid space. (c) The tumor is dissected from the mammillary body through the retro-carotid space, inferior to the posterior communicating artery (arrow head). A good “look up” operative view is established. (d) Internal decompression of the tumor from the retro-carotid space through the superior and inferior space of the posterior communicating artery (arrow head). ON: optic nerve, T: tumor, ICA: Internal carotid artery, M1: M1 portion of the middle cerebral artery, m: Mammillary body, A1: A1 portion of the anterior cerebral artery, P1: P1 portion of the posterior cerebral artery, P2: P2 portion of the posterior cerebral artery
Figure 7
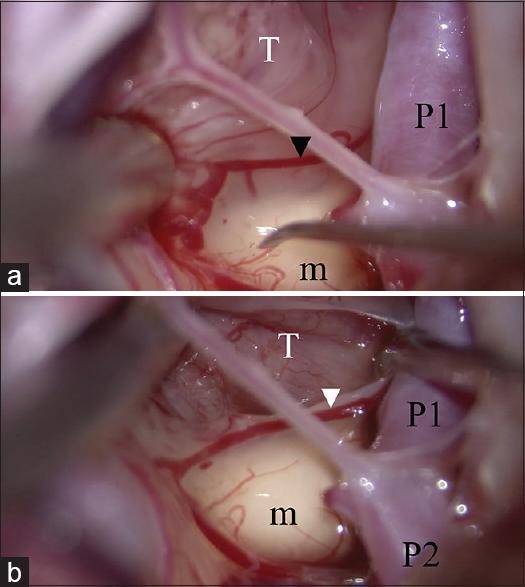
Identification of the cutting cleavage. (a) An inflammatory membrane covers both the tumor and normal tissue. The pial vessel (arrow head) belongs to the normal tissue. The dissecting plane is above the pial vessel. (b) The tumor capsule is identified and dissected from the normal structure. T: Tumor, m: Mammillary body, P1: P1 portion of the posterior cerebral artery, P2: P2 portion of the posterior cerebral artery
Figure 8
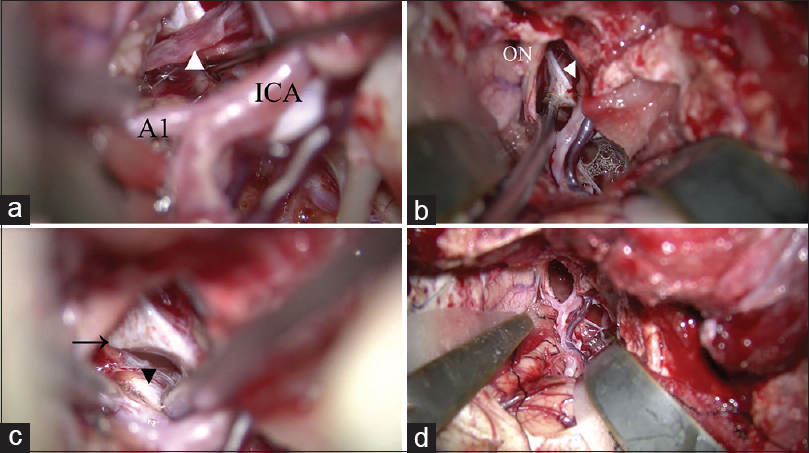
Removal of the tumor and identification of normal structures. (a) The pituitary stalk is identified as a thin compressed membrane (arrow head). (b) The distended diaphragma sellae is identified. (c) The contralateral posterior clinoid process (arrow) and oculomotor nerve (arrow head) are identified after removing the tumor. (d) All normal structures are secured after removal of the tumor. A1: A1 portion of the anterior cerebral artery, ICA: internal carotid artery, ON: optic nerve
RESULTS
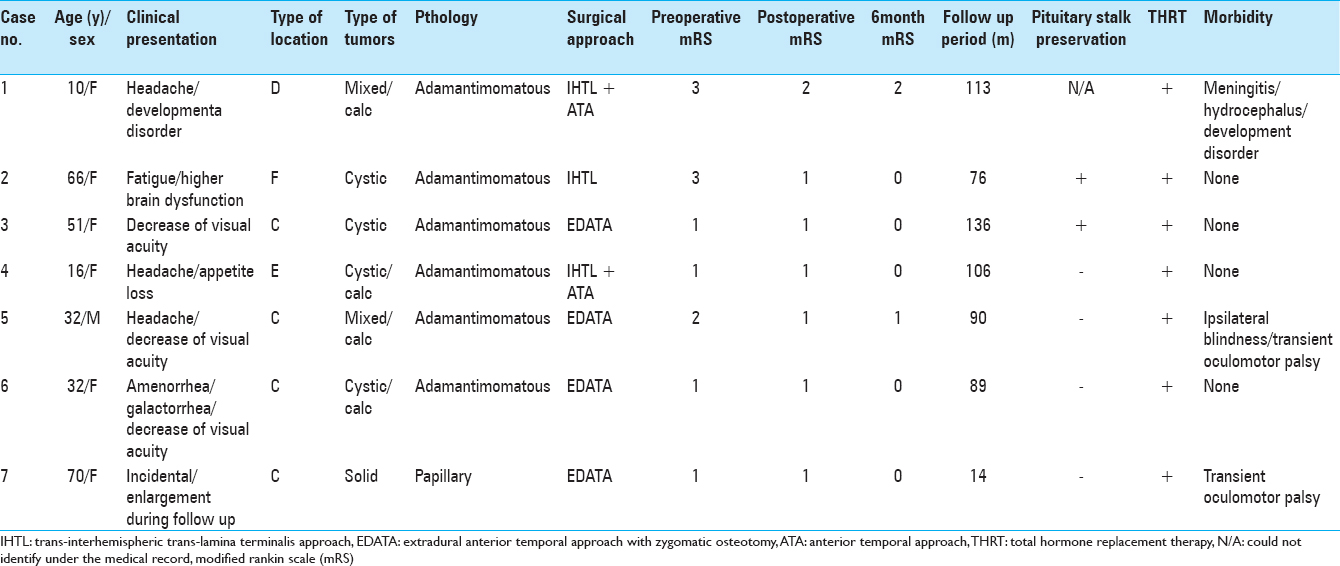
The preoperative modified Rankin scale (mRS) score was 1 in 4 patients, 2 in 1 patient, and 3 in 2 patients. The clinical presentation before the operation included decreased visual acuity in 3 patients, headache in 3, galactorrhea and amenorrhea in 1, higher brain dysfunction in 2, and developmental disorder in 1 patient. Diabetes insipidus was not apparent in any of the patients at the preoperative evaluation. The mean follow-up period was 89.1 months (14–136 months). No tumor recurrence was observed in these patients during the follow-up period. One patient had a papillary-type craniopharyngioma, whereas 6 had the adamantimomatous type [
The surgical approach was EDATA with zygomatic osteotomy in 4 patients, combined interhemispheric translamina terminalis approach and ATA in 2 patients, and interhemispheric translamina terminalis approach in 1 patient. Complete tumor resection was achieved in all 7 cases, with no recurrence during the follow-up period. The pituitary stalk was preserved in 2 cases.
The postoperative mRS score was 1 in 6 cases and 2 in 1 case. At the 6-month follow-up, the mRS score was 0 in 5 patients, 1 in 1 patient, and 2 in 1 patient. Transient morbidities included 2 cases of transient oculomotor nerve palsy on the operative side and 1 case of meningitis and hydrocephalus. The 2 cases of oculomotor nerve palsy improved within 3 months of the operation. There were 2 cases of permanent morbidity. One was that of hydrocephalus caused by meningitis and needing a ventriculoperitoneal shunt. The other was a case of blindness on the operative side. Preoperative ophthalmologic evaluation of the latter patient revealed typical bitemporal hemianopsia. Though the latter patient awoke from anesthesia with no deterioration in visual acuity and visual field, he lost vision suddenly 3 days after the operation. The ophthalmologic evaluation conducted at that time revealed no apparent abnormality in the anterior segment of the optic nerve or the retina and no optic nerve pallor. Posterior optic nerve neuropathy was diagnosed. In spite of corticosteroid administration, the visual acuity did not improve. The ophthalmic artery was found to be patent on CT angiography. The ophthalmologic evaluation at 2 weeks after the operation revealed right optic nerve atrophy and blindness. The left eye's temporal hemianopsia showed improvement.
Though the pituitary stalk was preserved in 2 cases, all 7 cases needed total hormone replacement therapy (HRT) with prednisolone, levothyroxine, and desmopressin. The visual acuity and visual field improved in 4 patients, 2 showed no change, and 1 showed a deterioration.
DISCUSSION
Radical resection in the first operation is important for treating craniopharyngiomas because the recurrence rate increases dramatically if the resection is incomplete and tumor resection is more difficult in successive operations than in the first one. Though many patients complain of panhypopituitarism after radical resection, with the advancements in the field of endocrinology, the various pituitary hormones such as antidiuretic hormone, cortisol, growth hormone, and levothyroxine can be supplied externally; the patient can even conceive by using HRT. In addition, the pituitary stalk can sometimes be preserved even if total resection is performed. Jung et al.[
Although the reported surgical approaches for this challenging tumor are divided into four categories, namely, the “look down” approach, “look up” approach, anterolateral approach, and trans-sphenoidal approach, the EDATA should be categorized as a lateral approach. The “look down” approaches include the interhemispheric translamina terminalis approach, the transcortical transventricular approach, and the transcallosal transventricular approach. The trans-sylvian (pterional) and the subfrontal trans-lamina terminalis approaches are categorized into the antero-lateral category. The orbitozygomatic approach is categorized as an antero-lateral and “look up” approach. We categorized EDATA as a lateral approach, and EDATA with zygomatic osteotomy as a lateral and “look up” approach. Because the difference between the anterolateral approach such as classical trans-sylvian approach and EDATA is wide operative window of the retrocarotid space. The intradural manipulations in the EDATA, such as dissection of the arachnoid trabeculae in between the uncus and the oculomotor nerve, and retraction of the temporal lobe posterolaterally, are similar to those in the anterior temporal approach. The anterior temporal approach,[
One limitation of this approach is its limited efficacy for intraventricular tumors. When the lamina terminalis is accessed via the lateral approach, the oblique trajectory of this route makes it difficult to visualize the posterior part of the third ventricle, especially the ipsilateral lateral wall of the hypothalamus.[
The reported complications associated with this approach are rhinorrhea or pneumocephalus due to opening of the paranasal sinus,[
Immediate visual disturbance after the operation can be because of heat injury, direct injury to the optic nerve, or occlusion of the ophthalmic artery. However, our patients experienced delayed visual disturbance. Rizzo et al.[
Other complications such as rhinorrhea and injury to the ICA can be avoided by strict preoperative anatomic evaluation and meticulous manipulation.[
CONCLUSION
EDATA with zygomatic arch osteotomy can ensure sufficient mobility of the ICA, and a good lateral and “look up” operative view. Hence, this is an effective approach for radical resection of craniopharyngiomas via the opticocarotid and retrocarotid spaces.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Avci E, Bademci G, Ozturk A. Microsurgical landmarks for safe removal of anterior clinoid process. Minim Invasive Neurosurg. 2005. 48: 268-72
2. Baskin DS, Wilson CB. Surgical management of craniopharyngiomas. A review of 74 cases. J Neurosurg. 1986. 65: 22-7
3. Behari S, Banerji D, Mishra A, Sharma S, Sharma S, Chhabra DK. Intrinsic third ventricular craniopharyngiomas: Report on six cases and a review of the literature. Surg Neurol. 2003. 60: 245-52
4. Ciric IS, Cozzens JW. Craniopharyngiomas: Transsphenoidal method of approach--For the virtuoso only?. Clin Neurosurg. 1980. 27: 169-87
5. Couldwell WT. Surgery of the anterior skull base. Otolaryngol Clin North Am. 1993. 26: 673-93
6. Day JD, Fukushima T, Giannotta SL. Cranial base approaches to posterior circulation aneurysms. J Neurosurg. 1997. 87: 544-54
7. Day JD, Giannotta SL, Fukushima T. Extradural temporopolar approach to lesions of the upper basilar artery and infrachiasmatic region. J Neurosurg. 1994. 81: 230-5
8. de Divitiis O, Angileri FF, d’Avella D, Tschabitscher M, Tomasello F. Microsurgical anatomic features of the lamina terminalis. Neurosurgery. 2002. 50: 563-9
9. Evans JJ, Hwang YS, Lee JH. Pre- versus post-anterior clinoidectomy measurements of the optic nerve, internal carotid artery, and opticocarotid triangle: A cadaveric morphometric study. Neurosurgery. 2000. 46: 1018-21
10. Fahlbusch R, Honegger J, Paulus W, Huk W, Buchfelder M. Surgical treatment of craniopharyngiomas: Experience with 168 patients. J Neurosurg. 1999. 90: 237-50
11. Gonzalez LF, Crawford NR, Horgan MA, Deshmukh P, Zabramski JM, Spetzler RF. Working area and angle of attack in three cranial base approaches: Pterional, orbitozygomatic, and maxillary extension of the orbitozygomatic approach. Neurosurgery. 2002. 50: 550-5
12. Heros RC, Lee SH. The combined pterional/anterior temporal approach for aneurysms of the upper basilar complex: Technical report. Neurosurgery. 1993. 33: 244-50
13. Honegger J, Buchfelder M, Fahlbusch R, Daubler B, Dorr HG. Transsphenoidal microsurgery for craniopharyngioma. Surg Neurol. 1992. 37: 189-96
14. Huynh-Le P, Natori Y, Sasaki T. Surgical anatomy of the anterior clinoid process. J Clin Neurosci. 2004. 11: 283-7
15. Jung TY, Jung S, Choi JE, Moon KS, Kim IY, Kang SS. Adult craniopharyngiomas: Surgical results with a special focus on endocrinological outcomes and recurrence according to pituitary stalk preservation. J Neurosurg. 2009. 111: 572-7
16. Kantarci M, Karasen RM, Alper F, Onbas O, Okur A, Karaman A. Remarkable anatomic variations in paranasal sinus region and their clinical importance. Eur J Radiol. 2004. 50: 296-302
17. Kaptain GJ, Vincent DA, Sheehan JP, Laws ER. Transsphenoidal approaches for the extracapsular resection of midline suprasellar and anterior cranial base lesions. Neurosurgery. 2001. 49: 94-100
18. Kato T, Sawamura Y, Abe H, Nagashima M. Transsphenoidal-transtuberculum sellae approach for supradiaphragmatic tumours: Technical note. Acta Neurochir. 1998. 140: 715-8
19. Katsuno M, Tanikawa R, Izumi N, Hashimoto M. A modified anterior temporal approach for low-position aneurysms of the upper basilar complex. Surg Neurol Int. 2015. 6: 10-
20. Kunishio K, Yamamoto Y, Sunami N, Asari S, Akagi T, Ohtsuki Y. Craniopharyngioma in the third ventricle: Necropsy findings and histogenesis. J Neurol Neurosurg Psychiatry. 1987. 50: 1053-6
21. Landolt AM, Zachmann M. Results of transsphenoidal extirpation of craniopharyngiomas and Rathke's cysts. Neurosurgery. 1991. 28: 410-5
22. Laws ER. Transsphenoidal microsurgery in the management of craniopharyngioma. J Neurosurg. 1980. 52: 661-6
23. Lemole GM, Henn JS, Zabramski JM, Spetzler RF. Modifications to the orbitozygomatic approach. Technical note. J Neurosurg. 2003. 99: 924-30
24. Liu JK, Cole CD, Kestle JR, Brockmeyer DL, Walker ML. Cranial base strategies for resection of craniopharyngioma in children. Neurosurg Focus. 2005. 18: E9-
25. Long DM, Chou SN. Transcallosal removal of cranio-pharyngiomas within the third ventricle. J Neurosurg. 1973. 39: 563-7
26. Maira G, Anile C, Albanese A, Cabezas D, Pardi F, Vignati A. The role of transsphenoidal surgery in the treatment of craniopharyngiomas. J Neurosurg. 2004. 100: 445-51
27. Maira G, Anile C, Colosimo C, Cabezas D. Craniopharyngiomas of the third ventricle: Trans-lamina terminalis approach. Neurosurgery. 2000. 47: 857-63
28. Matano F, Tanikawa R, Kamiyama H, Ota N, Tsuboi T, Noda K. Surgical treatment of 127 paraclinoid aneurysms with multifarious strategy: Factors related with outcome. World Neurosurg. 2016. 85: 169-76
29. Mikami T, Minamida Y, Koyanagi I, Baba T, Houkin K. Anatomical variations in pneumatization of the anterior clinoid process. J Neurosurg. 2007. 106: 170-4
30. Nutik SL. Removal of the anterior clinoid process for exposure of the proximal intracranial carotid artery. J Neurosurg. 1988. 69: 529-34
31. Ota N, Tanikawa R, Miyazaki T, Miyata S, Oda J, Noda K. Surgical microanatomy of the anterior clinoid process for paraclinoid aneurysm surgery and efficient modification of extradural anterior clinoidectomy. World Neurosurg. 2015. 83: 635-43
32. Otani N, Wada K, Kobayashi Y, Kumagai K, Ueno H, Osada H. Extradural temporopolar approach for surgical management of paraclinoid lesions. No Shinkei Geka. 2014. 42: 907-16
33. Rizzo JF. Visual loss after neurosurgical repair of paraclinoid aneurysms. Ophthalmology. 1995. 102: 905-10
34. Symon L, Pell MF, Habib AH. Radical excision of craniopharyngioma by the temporal route: A review of 50 patients. Br J Neurosurg. 1991. 5: 539-49
35. Yasargil MG, Curcic M, Kis M, Siegenthaler G, Teddy PJ, Roth P. Total removal of craniopharyngiomas. Approaches and long-term results in 144 patients. J Neurosurg. 1990. 73: 3-11
36. Yonekawa Y, Ogata N, Imhof HG, Olivecrona M, Strommer K, Kwak TE. Selective extradural anterior clinoidectomy for supra- and parasellar processes. Technical note. J Neurosurg. 1997. 87: 636-42
37. Zabramski JM, Kiris T, Sankhla SK, Cabiol J, Spetzler RF. Orbitozygomatic craniotomy. Technical note. J Neurosurg. 1998. 89: 336-41