- Service of Neurosurgery, Gabriel Montpied Hospital, University Hospital of Clermont-Ferrand, Clermont-Ferrand, France
- Image-Guided Clinical Neuroscience and Connectomics, Research Team, Auvergne University, Auvergne, France
- Biostatistics, Clinical Research Direction, Gabriel Montpied Hospital, University Hospital of Clermont-Ferrand, Clermont-Ferrand, France
- Service of Neurology, Gabriel Montpied Hospital, University Hospital of Clermont-Ferrand, Clermont-Ferrand, France
- Service of Radiology, Neuroradiology Unit, Gabriel Montpied Hospital, University Hospital of Clermont-Ferrand, Clermont-Ferrand, France
Correspondence Address:
Jean-Jacques Lemaire
Service of Neurology, Gabriel Montpied Hospital, University Hospital of Clermont-Ferrand, Clermont-Ferrand, France
DOI:10.4103/2152-7806.194066
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Jean-Jacques Lemaire, Bruno Pereira, Philippe Derost, François Vassal, Miguel Ulla, Dominique Morand, Guillaume Coll, Jean Gabrillargues, Ana Marques, Bérangère Debilly, Jérôme Coste, Franck Durif. Subthalamus stimulation in Parkinson disease: Accounting for the bilaterality of contacts. 14-Nov-2016;7:
How to cite this URL: Jean-Jacques Lemaire, Bruno Pereira, Philippe Derost, François Vassal, Miguel Ulla, Dominique Morand, Guillaume Coll, Jean Gabrillargues, Ana Marques, Bérangère Debilly, Jérôme Coste, Franck Durif. Subthalamus stimulation in Parkinson disease: Accounting for the bilaterality of contacts. 14-Nov-2016;7:. Available from: http://surgicalneurologyint.com/surgicalint_articles/subthalamus-stimulation-parkinson-disease-accounting-bilaterality-contacts/
Abstract
Background:Deep brain stimulation (DBS) in Parkinson's disease uses bi-hemispheric high-frequency stimulation within the subthalamus, however, the specific impacts of bilaterality of DBS are still not clear. Thus, we aimed to study the individual-level clinical impact of locations of right-left contact pair-up accounting for each subthalamic nucleus (STN) anatomy.
Methods:Contact locations and effects at 1 year were studied retrospectively in an unselected series of 53 patients operated between 2004 and 2010. Location of contacts was defined relatively to the main axis of STN used to map longitudinal and transversal positions, and STN membership (out meaning out-of-STN). Contact pairings were described via three methods: (i) Unified contact location (UCL) collapsing DBS into an all-in-one contact; (ii) balance of contact pair-up (BCPU), defined as symmetric or asymmetric regardless of laterality; (iii) hemisphere-wise most frequent contact pair-up (MFCP) regardless of BCPU. Clinical data were: mean levodopa equivalent dose, Unified Parkinson's Disease Rating Scale (UPDRS) motor score III without medication, UPDRS II and III speech sub-scores, UPDRS II freezing sub-score, 1 year versus preoperative values, with and without levodopa. Ad-hoc two-sided tests were used for statistical analysis.
Results:Worsening speech, was more frequent for UCL_out patients and when the left MFCP contact was rear and/or superolateral, however, it less frequent for BCPU-asymmetric patients. Worsening freezing was more frequent when the right MFCP contact was rear and superolateral.
Conclusions:These results point to strategies for minimizing dysarthria and freezing as adverse effects of DBS.
Keywords: Bilateral, deep brain stimulation, Parkinson's disease, Subthalamic nucleus
INTRODUCTION
Bilateral high-frequency chronic deep brain stimulation (DBS) of the subthalamic nucleus (STN) is an efficient treatment for motor complications in advanced Parkinson's disease[
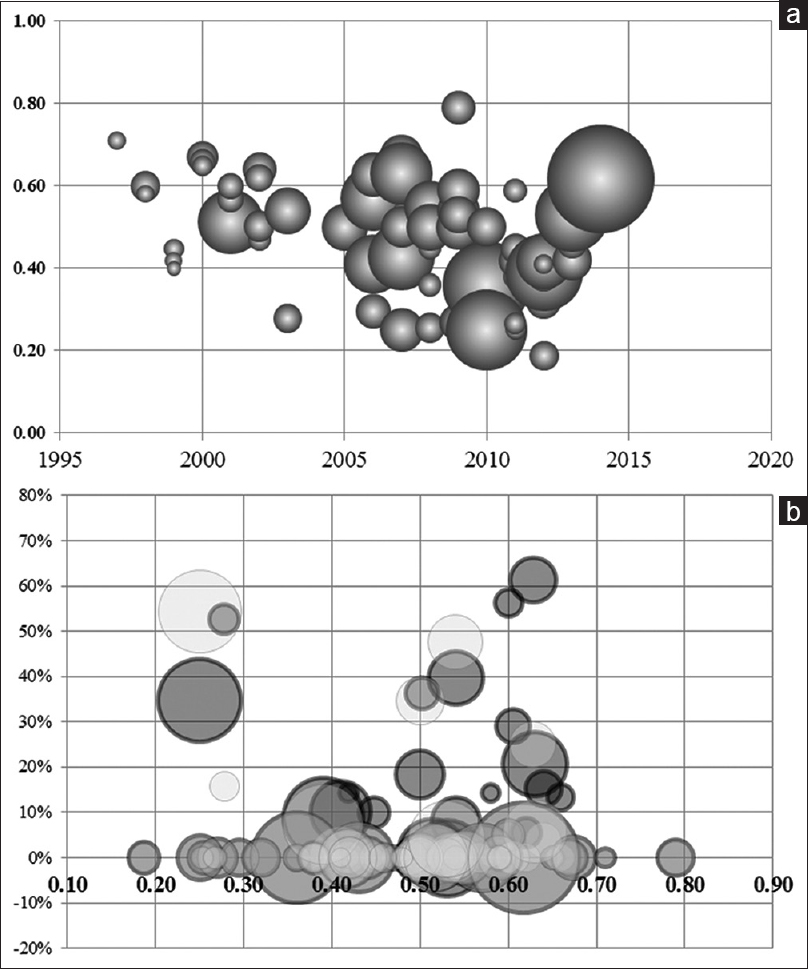
Figure 1
Overview of literature from 1994 to 2014: Bilateral, high-frequency, chronic deep brain stimulation (DBS) of the subthalamic nucleus (STN), or “STN DBS.” (a) Percent reduction of Unified Parkinson's Disease Rating Scale motor score III (y-axis) according to the year of publication; the circle size is proportional to number of patients in the series (n = 72). (b) Distribution of stimuli-induced adverse effects (y-axis; percentage of patients with speech worsening, grey circles, or postural worsening, white circles; circle size is proportional to number of patients in the series) according to the percent drop in Unified Parkinson's Disease Rating Scale motor score III without medication (x-axis) in the series (40 and 41 did not reported stimuli-induced adverse effects, respectively for speech and posture; out of 72)
We hypothesized that accounting for each individual right-left contact pair-up could be relevant to study either positive or adverse clinical effects for further personalization of electrode targeting and optimization of pulse settings. Here, we performed a single-center cross-sectional cohort study of 53 consecutive unselected patients, analyzing aggregate motor efficiency, dysarthria, and freezing according to the location of effective contacts used in chronic conditions at 1 year post-surgery. In addition, we analyzed the influence of age, gender, voltage, and drug modifications. We assumed an optimal compromise between medical treatment and bilateral DBS for each individual. Location of effective contacts was blinded from clinical results. Each right-left pair-up of effective contacts was specified according to STN landmarks, enabling two analyses, namely, a unified approach, where right-left contact pair-up was simplified, resulting in a unique location for each individual, and a bilateral approach, describing the balance of right-left contact pair-up for each patient and accounting for differences between the right and left hemispheric locations.
MATERIALS AND METHODS
Patients
Clinical data on 53 Parkinson's patients (two left-handed) operated consecutively between June 2004 and September 2010 were studied retrospectively after first securing Institutional Review Board approval. Bilateral subthalamic DBS (Lead 3387©, Kinetra©; Medtronic, USA) implantation was carried out according to the preoperative magnetic resonance imaging (MRI) anatomic mapping and intraoperative micro-recordings and clinical assessment (rigidity, tremor, speech) using semi-micro stimulation (MicroGuide Pro™; Alpha Omega, Israel) following an already published technique.[
Motor efficiency on the targeted motor symptoms was calculated on UPDRS III motor score, and expressed as percent improvement, in Dopa-OFF condition (MedOFF) at 1 year: StimOFF1yr – StimON1yr/StimOFF1yr; Dopa challenge, DopaOFF, 12 hours after withdrawal of antiparkinsonian drugs; StimOFF, 1 hour after turning off the stimulator; stimON, using the chronic parameters at 1 year (substantial clinical improvement; compromise with any adverse effects; optimized dopatherapy). Mean percent UPDRS III improvement in Dopa-OFF condition was 37.1% [
Table 2
Distribution of patients according to the modifications of Unified Parkinson's Disease Rating Scores, following bilateral subthalamic deep brain stimulation: option A, less sensitive to worsening; option B, more sensitive to worsening; (a) Unified Parkinson's Disease Rating Scores II speech sub-score; (b) Unified Parkinson's Disease Rating Scores III speech sub-score; (c) Unified Parkinson's Disease Rating Scores freezing sub-score
Right-plus-left 1-year effective contacts (n = 106) were: 10 times contact 0 (9.4%), 49 times contact 1 (46.2%), and 42 times contact 2 (39.6%), thus, 95.3% of contacts were within the subthalamus; and 5 times contact 3 (4.7%). The average (±SEM; median) and min–max 1-year voltage values (monopolar stimulation 102 times out of 106; 130 Hz) of right and left contacts were 2.92 V (±0.98; 2.80), 1.00–6.30 and 2.98 V (±0.87; 2.80), 1.30–6.30, respectively, with no significance difference between the two sides (P = 0.58, paired t-test). For further analysis, voltage difference, left minus right, and absolute value of difference for each individual (mean ± SEM; median; min-max) were calculated as: Left minus right = 0.06 V (±0.76; 0.00), min − 2 V, ma × 2.80 V; the absolute value of difference 0.44 V (±0.62; 0.20), min 0 V, ma × 2.80 V. Mean variation in levodopa equivalent drugs (LED) expressed as percent LED variation, i.e. preoperative dose – 1-year postoperative dose/preoperative dose (n = 50, 3 missing data) was 0.1 ± 0.5 (min = −1.7; max = 0.8), with a positive value indicating a drop in LED. For further analysis, the percentages of LED variation were segregated into three classes: <−30%, significant rise, 8 patients; [−30%, 30%], no significant change, 23 patients; >30%, significant drop, 19 patients.
Location of effective contacts according to subthalamic nucleus landmark
Location of effective contacts (chronic stimulation 1 year after electrode implantation) was determined for the right and left hemispheres. Each contact was identified on postoperative CT scan[
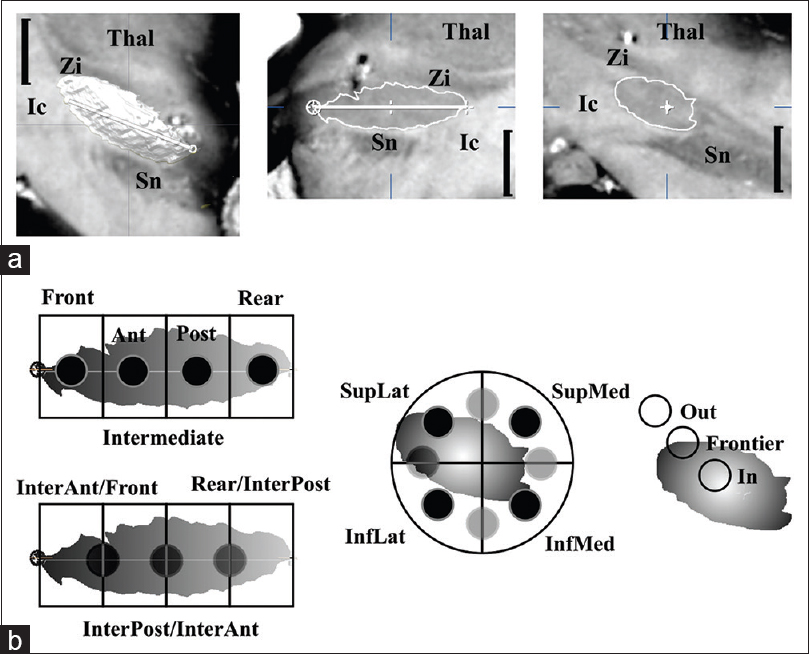
Figure 2
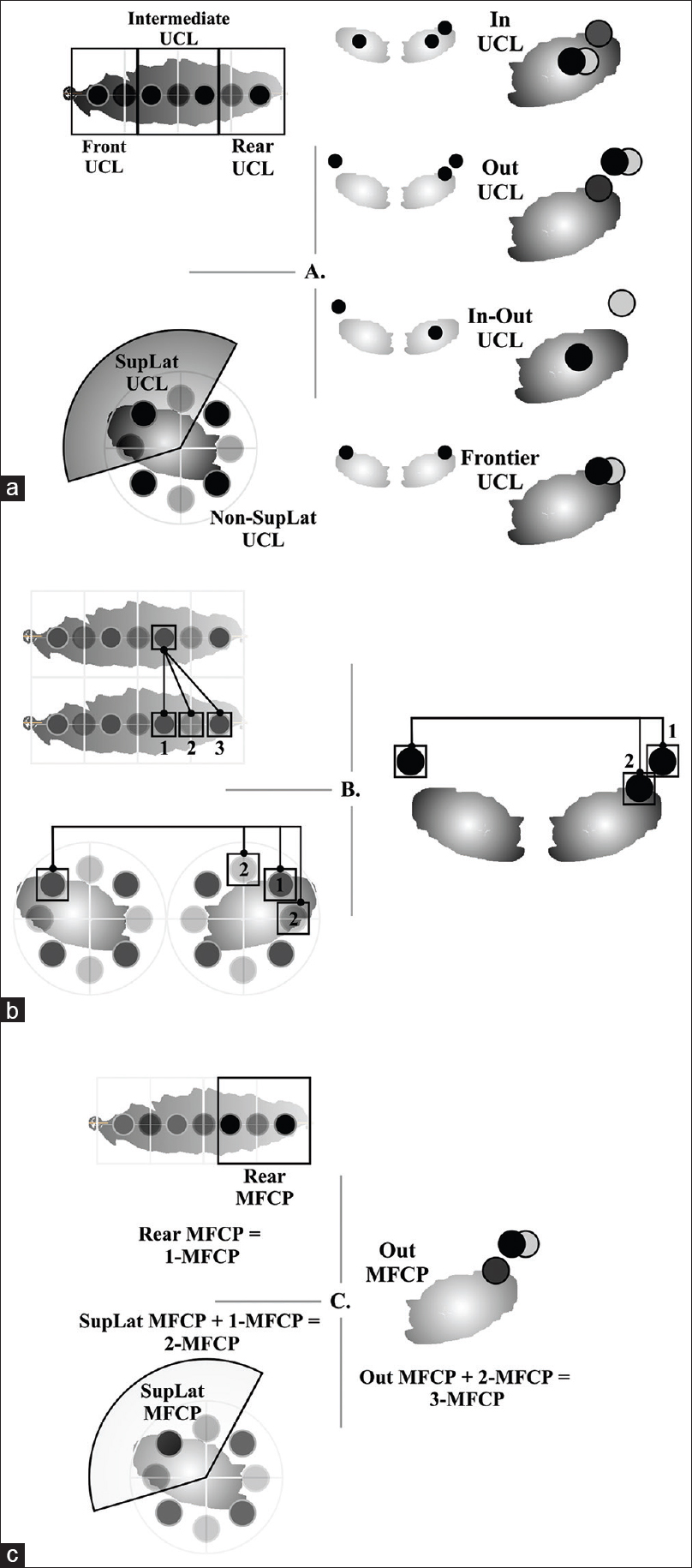
Subdivisions of the anatomic space centered on the subthalamic nucleus (STN). (a) Triplanar 4.7 T MRI of an anatomic specimen (black vertical bar = 5 mm): frontal view (left), anterior commissure-posterior commissure aligned, of the STN (3D, white) located below the thalamus (Thal) and zona incerta (Zi), above the substantia nigra (Sn), and medially to the internal capsule (Ic); longitudinal (intermediate) and transversal (right) sections running through the main axis of STN (white line) and the midpoint (white cross) of the longitudinal axis, respectively. (b) Longitudinal (left) and transversal (intermediate) subdivisions (primary locations, black dots; combined locations, gray dots) and STN membership (right) used to locate the effective contacts (see text for abbreviations)
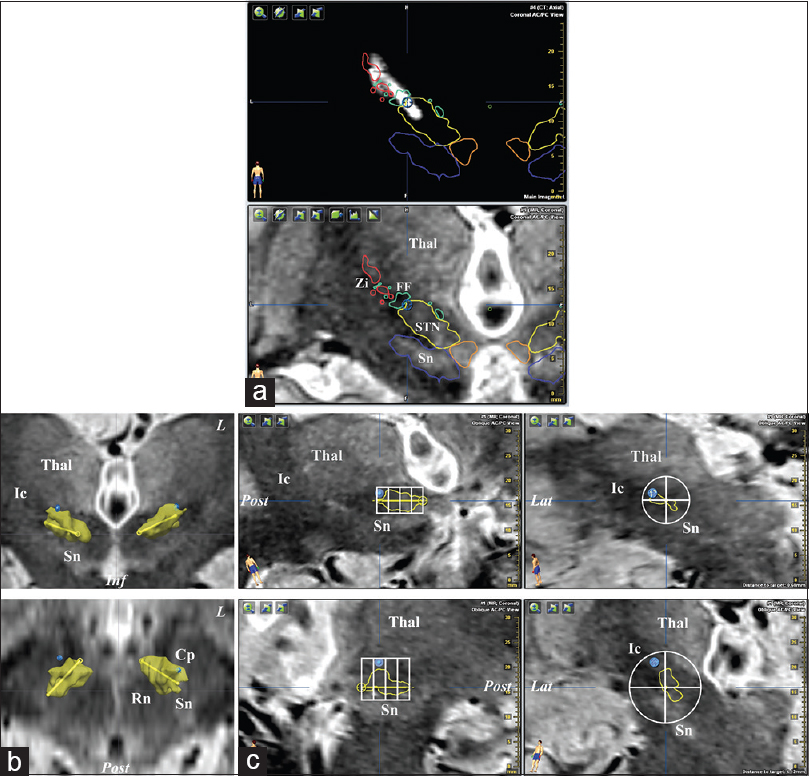
Figure 3
Example (patient #53, see
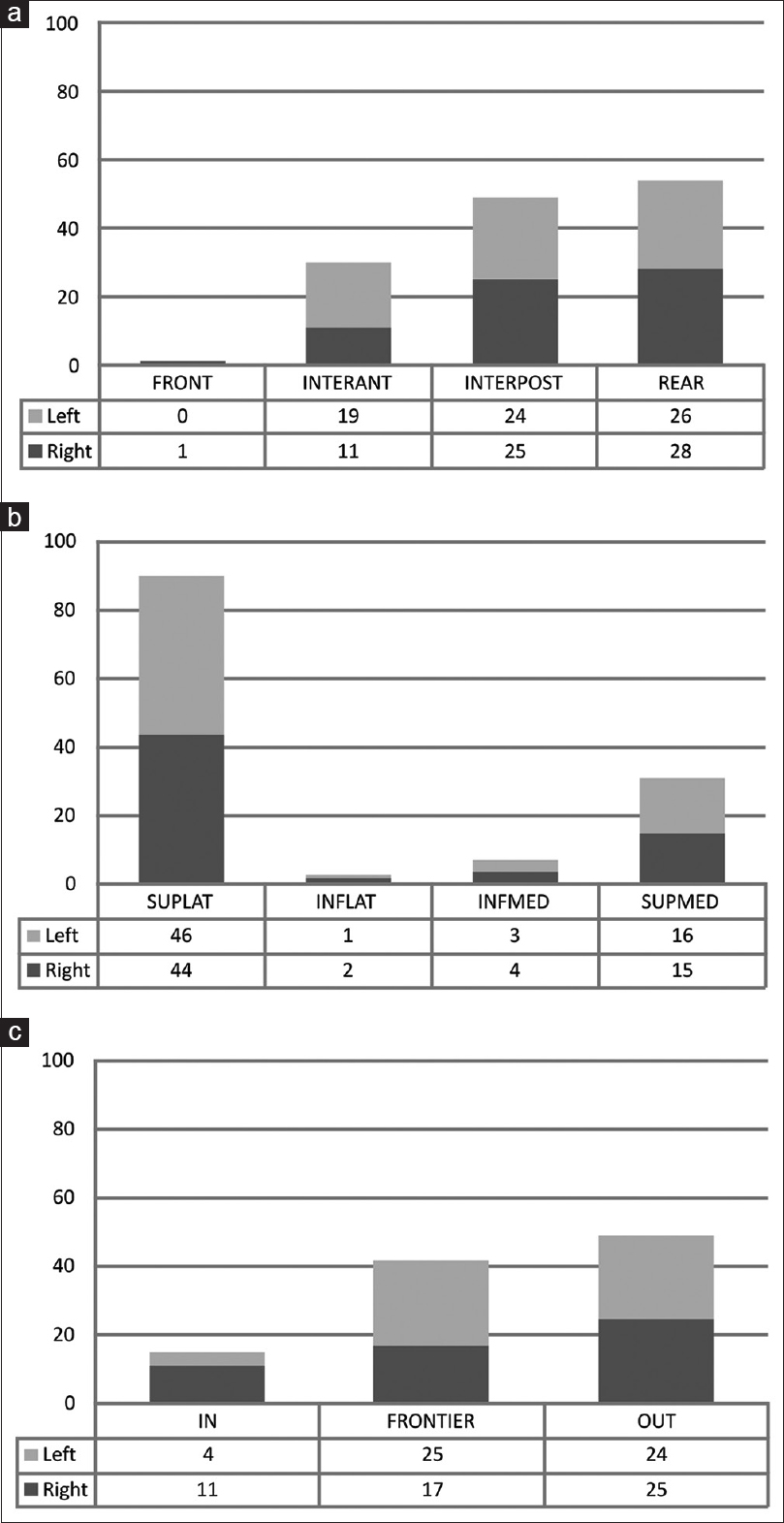
We defined a unified contact location (UCL) of right and left contacts for each patient because the so-called “STN DBS” unifies right and left contacts assuming no significant asymmetry. The 53 patients were regrouped according to simplified longitudinal (Front, Intermediate, and Rear) and transversal (SuperoLateral, SupLat UCL; Non-SuperoLateral, Non-SupLat UCL) locations (LonTranUCL) [
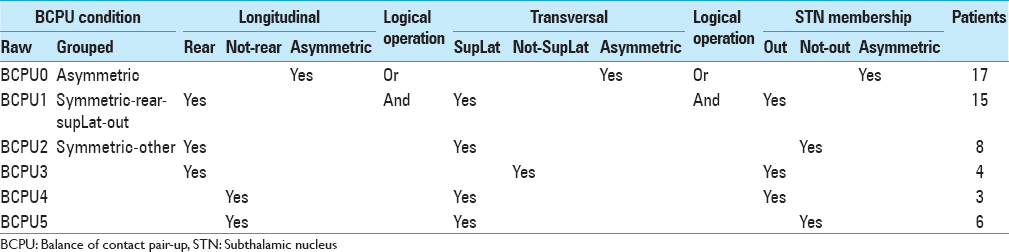
Balance of contact pair-up (BCPU) was defined as symmetric or asymmetric regardless of laterality (R-L BCPU was not differentiated from L-R BCPU), and was determined for longitudinal, transversal, and STN membership aspects [
We studied the left or right hemisphere-wise laterality of most frequent contact pair-ups (MFCP) regardless of either symmetric or asymmetric BCPU [Figure
Data analysis
Statistical analysis was performed using Stata software version 13 (StataCorp, College Station, TX). Data were presented as mean ± standard deviation (SD) or median (interquartile range) for continuous data and as number of patients and associated percentages for categorical parameters. Comparisons between independent groups were analyzed using the Chi-squared test or Fisher's exact test for categorical variables followed, when appropriate, by Marascuillo's procedure, and analysis of variance (ANOVA) or Kruskal–Wallis test for quantitative variables, with normality verified by the Shapiro–Wilk test and homoscedasticity verified by the Bartlett test. When appropriate, post-hoc multiple comparisons tests were proposed (Tukey-Kramer after ANOVA and Dunn for Kruskal-Wallis). Non-parametric tests were often preferred due to sample size. For paired comparisons, a paired t-test or Wilcoxon test was used for quantitative data and a Stuart–Maxwell test for qualitative parameters. All tests were two-sided, with a type-I error set at α = 0.05, without mathematical correction.[
RESULTS
We did not find differences in UPDRS III motor score, voltage, age, or gender according to UCL [Tables
We did not find differences in UPDRS III motor score, voltage, age, and gender according to BCPU [Tables
We did not find differences in voltage and age according to MFCP [Tables
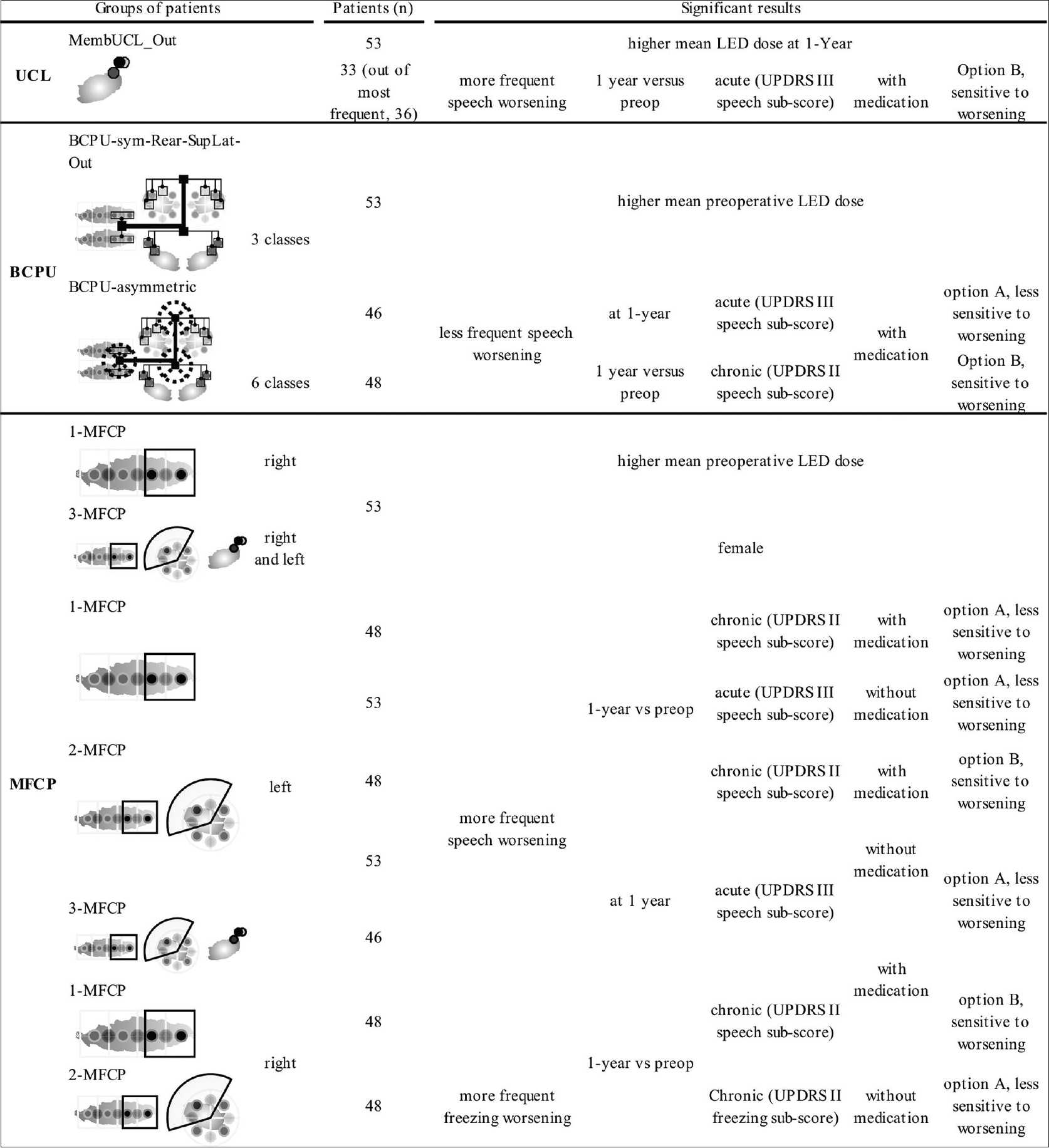
The full results are summarized in
DISCUSSION
Our results show that the precise location of effective contacts within the subthalamic region, regardless of the method used for location analysis, i.e. UCL, individual balance of contact pair-ups, or individual hemisphere-wise most frequent contact pair-up, does not explain the degree of motor improvement (UPDRS III). Consequently, the only key factor would be location within the subthalamic region, provided the contact is located within, at the frontier, or above the STN. These results are consistent with other studies reporting effective contact location, using different methods, at different locations in the subthalamic region (see
We also found that contacts located outside the STN seemed associated with more frequent speech worsening, regardless of the hemisphere and balance contact pair-up (acute test with medication versus preoperative scores). It appears as though electric stimulation plus dopamine functionally disrupts speech circuitry, specifically categorical fluency.[
In regards to freezing worsening, we observed that regardless of STN membership, patients with the right contact located posteriorly and laterally were more prone to worsening. We hypothesize that fibers ascending from the pedunculopontine nucleus (PPN) toward the substantia nigra compacta, STN, pallidum, and thalamus[
CONCLUSIONS
Right-left contact pair-up could be an important factor for optimization of DBS electrode targeting and electric stimulation parameter, in severe Parkinson's disease, and for efforts to gain a sharper understanding of the precise mechanisms of effects. Neurologists should aim to position right and left contacts asymmetrically to the STN landmark to minimize speech worsening. In particular, the left contact should not be in posterior, superior, and lateral position. The data reported here could be used for surgical targeting and proposed to neurologists as postoperative electrical settings if contacts are remotely selectable and anatomical location is known.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
SUPPLEMENTARY MATERIAL 2
Subthalamus deep brain stimulation in parkinson's disease: Accounting for the bilaterality of contacts
Acknowledgments
The authors thank Dr V. Mendes and Dr L. Ouchchane for their constructive comments during the very first phase of research project.
References
1. Benabid AL, Chabardes S, Mitrofanis J, Pollak P. Deep brain stimulation of the subthalamic nucleus for the treatment of Parkinson's disease. Lancet Neurol. 2009. 8: 67-81
2. Boertien TZrinzo LKahan JJahanshahi MHariz MMancini LLast accessed on 2011 July 15. Available: http://www.ncbi.nlm.nih.gov/pubmed/21674623.
3. Caire F, Derost P, Coste J, Bonny JM, Durif F, Frenoux E. Subthalamic deep brain stimulation for severe idiopathic Parkinson's disease. Location study of the effective contacts. Neurochirurgie. 2006. 52: 15-25
4. Caliandro P, Insola A, Scarnati E, Padua L, Russo G, Granieri E. Effects of unilateral pedunculopontine stimulation on electromyographic activation patterns during gait in individual patients with Parkinson's disease. J Neural Transm. 2011. 118: 1477-86
5. Campbell MC, Black KJ, Weaver PM, Lugar HM, Videen TO, Tabbal SD. Mood response to deep brain stimulation of the subthalamic nucleus in Parkinson's disease. J Neuropsychiatry Clin Neurosci. 2012. 24: 28-36
6. Coste J, Ouchchane L, Sarry L, Derost P, Durif F, Gabrillargues J. New electrophysiological mapping combined with MRI in parkinsonian's subthalamic region. Eur J Neurosci. 2009. 29: 1627-33
7. Falowski SM, Sharan A, Reyes BA, Sikkema C, Szot P, Van Bockstaele EJ. An evaluation of neuroplasticity and behavior after deep brain stimulation of the nucleus accumbens in an animal model of depression. Neurosurgery. 2011. 69: 1281-90
8. Fasano A, Daniele A, Albanese A. Treatment of motor and non-motor features of Parkinson's disease with deep brain stimulation. Lancet Neurol. 2012. 11: 429-42
9. Fenoy AJ, Goetz L, Chabardès S, Xia Y. Deep brain stimulation: Are astrocytes a key driver behind the scene?. CNS Neurosci Ther. 2014. 20: 191-201
10. Guehl D, Vital A, Cuny E, Spampinato U, Rougier A, Bioulac B. Postmortem proof of effectiveness of zona incerta stimulation in Parkinson disease. Neurology. 2008. 70: 1489-90
11. Hariz M. Deep brain stimulation: New techniques. Parkinsonism Relat Disord. 2014. 20: S192-6
12. Haynes WI, Haber SN. The organization of prefrontal-subthalamic inputs in primates provides an anatomical substrate for both functional specificity and integration: Implications for Basal Ganglia models and deep brain stimulation. J Neurosci. 2013. 33: 4804-14
13. Hemm S, Coste J, Gabrillargues J, Ouchchane L, Sarry L, Caire F. Contact position analysis of deep brain stimulation electrodes on post-operative CT images. Acta Neurochir. 2009. 151: 823-9
14. Hill KK, Campbell MC, McNeely ME, Karimi M, Ushe M, Tabbal SD. Cerebral blood flow responses to dorsal and ventral STN DBS correlate with gait and balance responses in Parkinson's disease. Exp Neurol. 2013. 241: 105-12
15. Hilliard JD, Frysinger RC, Elias WJ. Effective subthalamic nucleus deep brain stimulation sites may differ for tremor, bradykinesia and gait disturbances in Parkinson's disease. Stereotact Funct Neurosurg. 2011. 89: 357-64
16. Karlsson F, Olofsson K, Blomstedt P, Linder J, van Doorn J. Pitch variability in patients with Parkinson's disease: Effects of deep brain stimulation of caudal zona incerta and subthalamic nucleus. J Speech Lang Hear Res. 2013. 56: 150-8
17. Lalys F, Haegelen C, Mehri M, Drapier S, Vérin M, Jannin P. Anatomo-clinical atlases correlate clinical data and electrode contact coordinates: Application to subthalamic deep brain stimulation. J Neurosci Methods. 2013. 212: 297-307
18. Lemaire JJ, Coste J, Ouchchane L, Caire F, Nuti C, Derost P. Brain mapping in stereotactic surgery: A brief overview from the probabilistic targeting to the patient-based anatomic mapping. Neuroimage. 2007. 37: S109-15
19. Lemaire J-J, Cosnard G, Sakka L, Nuti C, Gradkowski W, Mori S. White matter anatomy of the human deep brain revisited with high resolution DTI fibre tracking. Neurochirurgie. 2011. 57: 52-67
20. Moro E, Hamani C, Poon YY, Al-Khairallah T, Dostrovsky JO, Hutchison WD. Unilateral pedunculopontine stimulation improves falls in Parkinson's disease. Brain. 2009. 133: 215-24
21. Nieuwenhuys R, Voogd J, Huijzen C.editors. The human central nervous system: A synopsis and atlas. Berlin: Springer-Verlag; 1979. p.
22. Obeso JA, Rodríguez-Oroz MC, Benitez-Temino B, Blesa FJ, Guridi J, Marin C. Functional organization of the basal ganglia: Therapeutic implications for Parkinson's disease. Mov Disord. 2008. 23: S548-59
23. Okun MS. Deep-brain stimulation for Parkinson's disease. N Engl J Med. 2012. 367: 1529-38
24. Okun MS. Deep-brain stimulation for Parkinson's disease. N Engl J Med. 2013. 368: 483-4
25. Parent A, Côté PY, Lavoie B. Chemical anatomy of primate basal ganglia. Prog Neurobiol. 1995. 46: 131-97
26. Parent A, Hazrati LN. Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res Brain Res Rev. 1995. 20: 91-127
27. Plaha P, Ben-Shlomo Y, Patel NK, Gill SS. Stimulation of the caudal zona incerta is superior to stimulation of the subthalamic nucleus in improving contralateral parkinsonism. Brain. 2006. 129: 1732-47
28. Pötter-Nerger M, Volkmann J. Deep brain stimulation for gait and postural symptoms in Parkinson's disease. Mov Disord. 2013. 28: 1609-15
29. Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990. 1: 43-6
30. Schuepbach WMM, Rau J, Knudsen K, Volkmann J, Krack P, Timmermann L. Neurostimulation for Parkinson's disease with early motor complications. N Engl J Med. 2013. 368: 610-22
31. Sjöberg RL, Lidman E, Häggström B, Hariz MI, Linder J, Fredricks A. Verbal fluency in patients receiving bilateral versus left-sided deep brain stimulation of the subthalamic nucleus for Parkinson's disease. J Int Neuropsychol Soc. 2012. 18: 606-11
32. Temel Y, Blokland A, Steinbusch HW, Visser-Vandewalle V. The functional role of the subthalamic nucleus in cognitive and limbic circuits. Prog Neurobiol. 2005. 76: 393-413
33. Tripoliti E, Zrinzo L, Martinez-Torres I, Frost E, Pinto S, Foltynie T. Effects of subthalamic stimulation on speech of consecutive patients with Parkinson disease. Neurology. 2011. 76: 80-6
34. Tripoliti E, Zrinzo L, Martinez-Torres I, Tisch S, Frost E, Borrell E. Effects of contact location and voltage amplitude on speech and movement in bilateral subthalamic nucleus deep brain stimulation. Mov Disord. 2008. 23: 2377-83
35. Voges J, Volkmann J, Allert N, Lehrke R, Koulousakis A, Freund HJ. Bilateral high-frequency stimulation in the subthalamic nucleus for the treatment of Parkinson disease: Correlation of therapeutic effect with anatomical electrode position. J Neurosurg. 2002. 96: 269-79
36. Volkmann J, Moro E, Pahwa R. Basic algorithms for the programming of deep brain stimulation in Parkinson's disease. Mov Disord. 2006. 21: S284-9
37. Welter ML, Schüpbach M, Czernecki V, Karachi C, Fernandez-Vidal S, Golmard JL. Optimal target localization for subthalamic stimulation in patients with Parkinson disease. Neurology. 2014. 82: 1352-61
38. Yelnik J, Bardinet E, Dormont D, Malandain G, Ourselin S, Tandé D. A three-dimensional, histological and deformable atlas of the human basal ganglia. I. Atlas construction based on immunohistochemical and MRI data. Neuroimage. 2007. 34: 618-38
39. Zonenshayn M, Sterio D, Kelly PJ, Rezai AR, Beric A. Location of the active contact within the subthalamic nucleus (STN) in the treatment of idiopathic Parkinson's disease. Surg Neurol. 2004. 62: 216-25