- Department of Endocrinology, Post Graduate Institute of Medical Education and Research, Chandigarh, India.
- Department of Neurosurgery, Post Graduate Institute of Medical Education and Research, Chandigarh, India.
- Department of Histopathology Post Graduate Institute of Medical Education and Research, Chandigarh, India.
- Department of Radiodiagnosis, Post Graduate Institute of Medical Education and Research, Chandigarh, India.
Correspondence Address:
Pinaki Dutta
Department of Endocrinology, Post Graduate Institute of Medical Education and Research, Chandigarh, India.
DOI:10.25259/SNI_877_2020
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Aditya Dutta1, Nimisha Jain1, Ashutosh Rai1, Rahul Gupta1, Sivashanmugam Dhandapani2, Anil Bhansali1, Bishan Das Radotra3, Chirag Kamal Ahuja4, Pinaki Dutta1. The outcome of TSHoma from a tertiary care institute in India. 14-Apr-2021;12:161
How to cite this URL: Aditya Dutta1, Nimisha Jain1, Ashutosh Rai1, Rahul Gupta1, Sivashanmugam Dhandapani2, Anil Bhansali1, Bishan Das Radotra3, Chirag Kamal Ahuja4, Pinaki Dutta1. The outcome of TSHoma from a tertiary care institute in India. 14-Apr-2021;12:161. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=10711
Abstract
Background: Thyroid-stimulating hormone (TSH)-secreting pituitary adenoma (TSHoma) is the rarest functioning pituitary adenoma.
Methods: A retrospective analysis of eight patients of TSHomas to highlight the presentations, diagnostic challenges, and treatment outcomes.
Results: Median age at diagnosis was 42 years, median latency to diagnosis was 2.5 years, and thyrotoxic and compressive symptoms were the most common presenting symptoms. At presentation, three cases were plurihormonal, six cases were on medical treatment including thyroxine, and two cases were incidentally discovered. Imaging revealed macroadenoma in all cases. Seven cases underwent pituitary surgery, after which three achieved remission. Another case entered remission after adjunctive radiotherapy. Thyrotropin (TSH) immunostaining was demonstrated in six out of seven adenomas.
Conclusion: TSHoma is a rare functioning pituitary tumor with both silent and symptomatic presentations. Diagnosis can be established with biochemical and imaging features, even without dynamic tests.
Keywords: Non-suppressed thyroid-stimulating hormone, Secondary hyperthyroidism, Thyroid-stimulating hormone-secreting pituitary adenoma
INTRODUCTION
Thyroid-stimulating hormone (TSH)-secreting pituitary adenoma (TSHoma) is the rarest functioning pituitary adenoma. Despite being functional, late diagnosis, or misdiagnosis is the rule. The patient usually presents with compressive symptoms of headache, visual field defects, and multiple pituitary hormone deficiencies (MPHD).[
MATERIALS AND METHODS
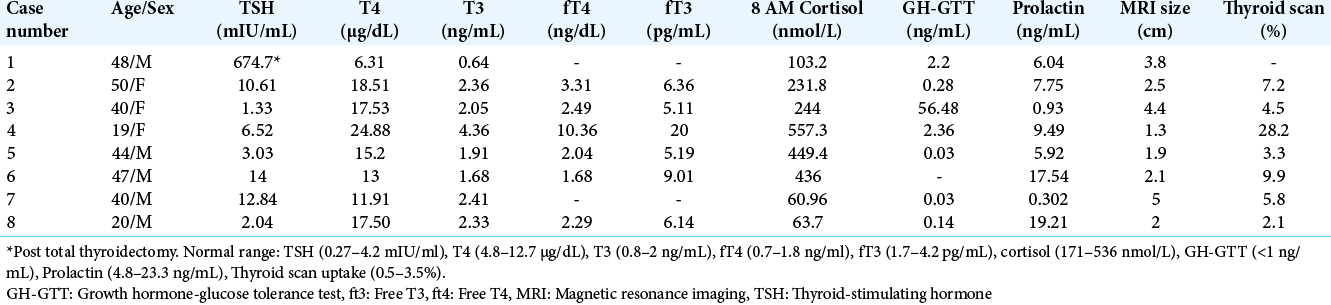
This is a retrospective, record-based study of eight cases with biochemically, radiologically, and histologically (all but one) verified TSHoma that presented at Post Graduate Institute of Medical Education and Research, Chandigarh, from the year 2012 to 2019. During this period, approximately 1500 pituitary surgeries were performed. The study was approved by the Institute Ethics Committee and informed written consent was obtained from all the study participants. Baseline characteristics are summarized in [
CASES
Case 1
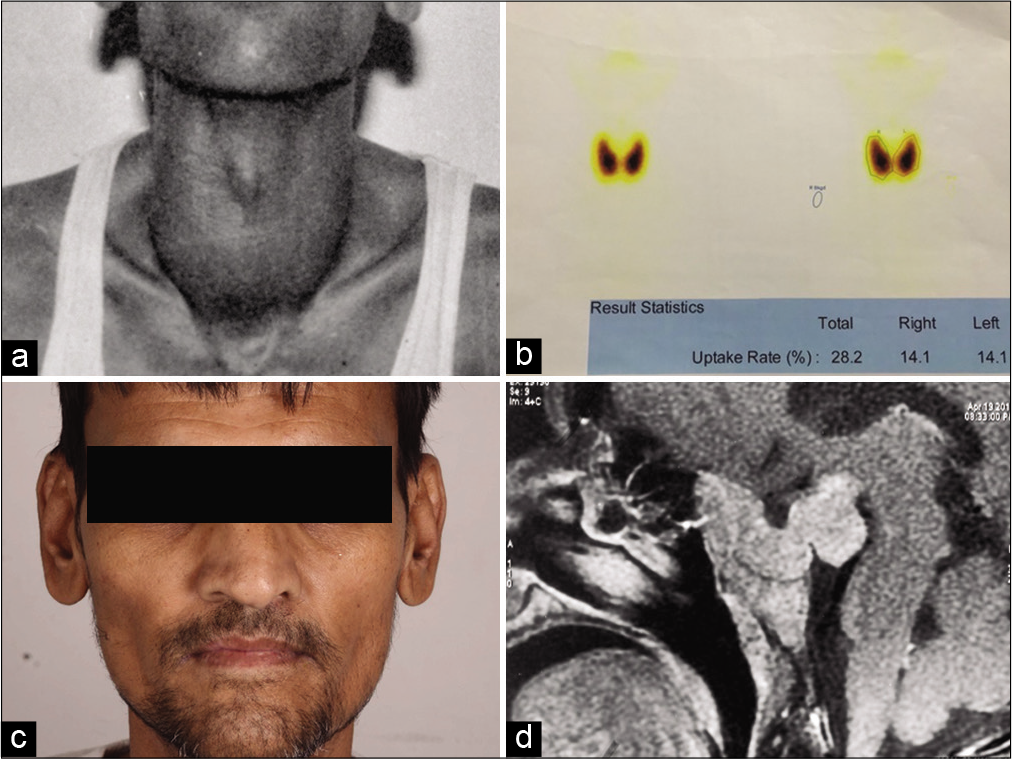
A 48-year-old male presented with history of total thyroidectomy for diffuse toxic goiter 10-years ago [
Case 2
A 48-year-old female presented with secondary amenorrhea, decreased vision, and headache. A TFT done 2-years earlier showed TSH 25.4 mIU/mL, T4 4.02 μg/dL, and T3 0.66 ng/mL. Even after initiating thyroxine (100 μg), her TSH never normalized. Palpitations, tremor, hair loss, heat intolerance, sweating, systolic hypertension, and Grade II goiter were present, and TFT (on thyroxine 250 μg) was as follows: TSH 10.61 mIU/mL, T4 18.51 μg/dL, fT4 3.31 ng/dL, T3 2.36 ng/mL, fT3 6.36 pg/mL, and anti-TPO 356.6 IU/mL (<60). The patient remained thyrotoxic despite stopping thyroxine. CEMRI sella revealed a 2.2*1.8*2.5 cm sellarsuprasellar mass with para-sellar extension. VFC showed bi-temporal hemianopia. Ultrasound neck revealed thyromegaly and thyroid scintigraphy revealed increased trapping function in both lobes of the thyroid (7.2%). The patient was started on carbimazole 30 mg daily, octreotide LAR 30 mg once a month, and underwent TSS 3 months later. Postoperatively her TSH remained elevated. There were no complications of the surgery. Histopathology confirmed pituitary adenoma and IHC was positive for TSH in 80% cells. For residual adenoma (size: 1.4*1.4*0.9 cm), the patient underwent gamma-knife radio-surgery (GKRS) and achieved remission 1 year later.
Case 3
A 40-year-old female presented with secondary amenorrhea, headache, enlargement of hands and feet, and decreased vision. Although clinically diagnosed as acromegaly, thyrotoxic symptoms (heat intolerance, tremor, and palpitations) and Grade Ib goiter were present. Hormone analysis was as follows: TSH 1.33 mIU/mL, T4 17.53 μg/dL, fT4 2.49 ng/dL, T3 2.05 ng/mL, fT3 5.11 pg/mL, and nadir GH 56.48 ng/mL on GTT. CEMRI sella revealed 4.4*3.8*3.4 cm sellar mass with supra-sellar and para-sellar extensions. VFC showed bi-temporal hemianopia. Thyroid scan revealed increased trapping function in both lobes of the thyroid (4.5%). The patient was started on carbimazole 30 mg daily and octreotide LAR 30 mg once a month, and underwent TSS with near-total resection 3 months later. Surgical complications included CSF rhinorrhea and DI. Although GH remained elevated postoperatively, TSH became undetectable, and T4 normalized. Histopathology showed adenoma; however, IHC showed 90% positivity for GH and scattered positivity for TSH. Further IHC analysis revealed ubiquitous PIT1 expression. IHC for GATA-2 could not be done due to its unavailability.
Case 4
A 19-year-old female presented with thyrotoxicosis (weight loss, sweating, palpitations, increased appetite, tremor, hair loss, anxiety, and heat intolerance) and Grade II goiter. Baseline TFT was: TSH 8.79 mIU/mL, T4 26.99 μg/dL, fT4 10.36 ng/dL, T3 5.20 ng/mL, and fT3 20 pg/mL. Despite being on carbimazole 40 mg for >1 year, there was no improvement in symptoms. At presentation TFT was: TSH 6.52 mIU/mL, T4 24.88 μg/dL, and T3 of 4.36 ng/mL. Although clinical features of acromegaly were lacking, the patient had nadir GH of 2.36 ng/mL on GTT and elevated Insulin-like growth factor-1 (IGF-1) levels (909 ng/mL). CEMRI brain showed a 1.3*1.0 cm sellarsuprasellar mass. Thyroid scan revealed increased trapping function in both lobes of the thyroid (28.2%) [
Figure 2:
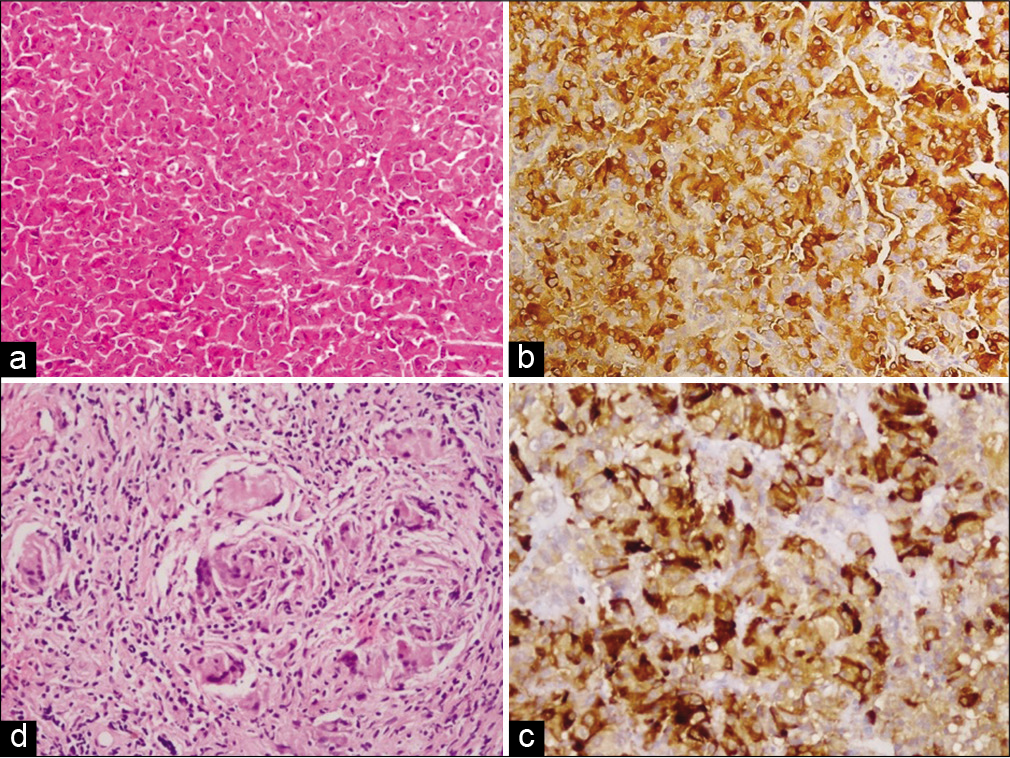
(a and b) Histopathology (20×) depicting monomorphic adenoma cells with abundant cytoplasm, arranged in sheet and nest such as pattern and diffuse (80%) thyroid-stimulating hormone (TSH) stain positivity in TSH-secreting pituitary adenoma of case 7; (c and d) 30% TSH stain positivity in adenoma cells and meningeal biopsy (20×) showing multiple granuloma (fungal) in Case 8.
Case 5
A 44-year-old male presented with headache. A local physician advised a CEMRI brain which revealed a 1.6*1.9*1.4 cm pituitary macroadenoma. Hormone analysis was normal except for deranged TFT: TSH 3.03 mIU/mL, T4 15.2 μg/dL, fT4 2.04 ng/dL, T3 1.91 ng/mL, and fT3 5.19 pg/mL. There were no clinical features of thyrotoxicosis or goiter. Thyroid scan revealed borderline increased trapping function in both lobes of the thyroid (3.3%). The patient underwent TSS and had surgical complications of secondary hypocortisolism, CSF rhinorrhea, and transient DI. Postoperatively, the patient achieved remission. Histopathology was consistent with adenoma and IHC revealed 30% positivity for TSH. The patient presented 1-month later with sudden-onset high-grade fever, generalized tonic-clonic seizure, and altered sensorium. The patient was diagnosed to have acute bacterial meningitis and succumbed to the illness.
Case 6
A 47-year-old male presented with headache, weight loss, palpitations, sweating, increased appetite, tremors, fatigue, and hypertension. On basis of TSH of 10.9 mIU/mL and fT4 of 2.07 ng/dL, carbimazole was initiated, however, both symptoms and TSH never normalized. At presentation TFT was: TSH 6.80 mIU/mL, fT4 5.3 ng/dL, and fT3 16.6 pg/mL. CEMRI sella revealed a 2.1*1.9*1.8 cm macroadenoma with para-sellar extension. Grade II goiter was present. VFC was normal. Thyroid scan showed increased trapping function in both lobes of the thyroid (9.9%). The patient underwent TSS without any surgical complications. Histopathology was consistent with adenoma with IHC showing positivity for TSH in 30% cells. The patient entered clinical remission, however, TSH remains detectable.
Case 7
A 40-year-old male presented with symptoms of headache and weight loss (40 kg). There was a history of uncontrolled Type 2 diabetes with palpitations, sweating, increased frequency of stools, fatigue, tremors, heat intolerance, loss of libido, and regression of secondary sexual characteristics [
Case 8
A 20-year-old male presented with headache and recurrent loss of consciousness. A CEMRI done elsewhere revealed a sellar mass 1.7*2.0*1.3 cm along with diffuse meningeal enhancement for which a CSF analysis was performed suggesting chronic meningitis. The patient was referred with a diagnosis of non-functioning pituitary adenoma and tubercular meningitis. Clinically, features of hypogonadism (poor virilization, testicular volume 3 mL) were present. Hormone analysis was as follows: TSH 2.04 mIU/mL, T4 17.5 μg/dL, fT4 2.29 ng/dL, T3 2.33 ng/mL, fT3 6.14 pg/mL, and elevated gonadotrophins (FSH > LH). Both ultrasound neck and thyroid scan were normal. In view of long leggedness, small testes, and elevated gonadotrophins, karyotyping was done which revealed 46, XXY. Repeat CSF analysis revealed 40 cells (all lymphocytes) and elevated proteins (114 mg/dL). With a diagnosis of TSHoma and chronic meningitis of unknown etiology, the patient underwent TSS and meningeal biopsy. Postoperatively, patient developed transient DI. Histopathology was consistent with adenoma; IHC showed positivity for TSH in 30% cells [
RESULTS
Patient characteristics
Patients were middle-aged (median: 42 years; range 19–50) with a slight male preponderance (5:3). Median latency to diagnosis was 2.5 years (range 0.17–11). Symptomatic thyrotoxicosis (5/8) and headache (6/8) were the most common modes of presentation. Other symptoms included visual field defects (4/8), hypogonadism (5/8), and acromegaly (1/8). The diagnosis was incidental in two cases. On examination, goiter was present in 5/8 cases. Six patients received prior treatment in the form of thyroxine (3/6), anti-thyroid drugs (4/6), and total thyroidectomy (1/6). No patient received thyroid ablation with radio-iodine.
Biochemical findings
Irrespective of the prior treatment modality, 7/8 cases were biochemically thyrotoxic at presentation. Median TSH (Case 1 excluded due to post thyroidectomy status) was 6.52 mIU/mL (range 0.69–12.84). In individual cases, TSH was inappropriately high for the corresponding thyroid hormone levels. Median T4 and T3 were 14.96 μg/dL (range 6.31–24.88) and 2.2 ng/mL (range 0.64–4.36), respectively [
Radiological findings
CEMRI sella revealed a macroadenoma in all eight cases. The median largest diameter was 2.3 cm (range 1.3–5). KNOSP grade was 3–4 in all cases. Ultrasonography neck confirmed thyromegaly in all cases where goiter was clinically detected. 99mTc pertechnatate thyroid scintigraphy revealed increased trapping function in both lobes of the thyroid in all cases (and no residual thyroid tissue in Case 1).
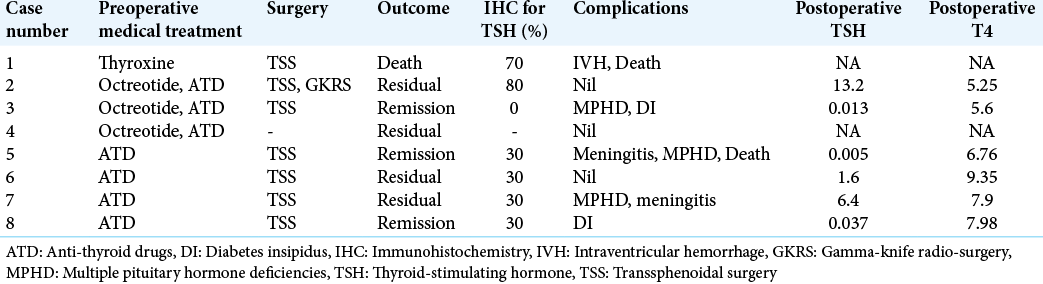
Preoperative medical preparation
Three cases received octreotide LAR before surgery. All cases received anti-thyroid drugs, Lugol’s iodine, and propranolol a few days before surgery to prevent thyroid storm.
Treatment and complications
Excluding Case 4 (declined surgery), all patients underwent TSS as the primary modality of treatment. Cases 2 and 7 received radiation therapy within a year of surgery. Postoperative complications included CSF leak, MPHD (new and persistent), acute meningitis, and transient or permanent DI. Case 1 developed intraventricular hemorrhage which resulted in his death. Case 5 developed meningitis after 1 month of discharge and later succumbed to the illness.
Outcomes
TFT was obtained within 1–2 weeks of surgery. Median postoperative TSH was 0.82 mIU/mL (range 0.005–13.2) and T4 was 7.14 μg/dL (range 5.25–9.35) [
Histology
Among the seven operated cases, pituitary adenoma was confirmed in all. Positive immunostaining for TSH was seen in 6/7 resected specimens (range 30–80%). Case 3 had diffuse positivity for GH and scattered positivity for TSH. Although three cases had plurihormonal presentation, IHC positivity for both GH and TSH could not be demonstrated. Ki-67 was <1% in both Cases 2 and 7, despite their large and invasive nature on imaging and dural invasion on histology.
DISCUSSION
The results of our study suggest that combined assessment of clinical features (thyrotoxicosis, goiter, and compressive symptoms), biochemical tests (non-suppressed TSH with elevated free thyroid hormones), and appropriate imaging is necessary for the diagnosis of TSHoma. The results also show that mismanagement with thyroxine or ATDs is a rule rather than an exception and that TSHoma can be pluri-hormonal.
Graves’ disease is the most common cause of hyperthyroidism overall. Secondary hyperthyroidism was first recognized in 1960 when a case of Graves’ disease had remission following radiotherapy for a pituitary neoplasm. It was only after the advent of the radioimmunoassay for TSH that the syndrome of inappropriate secretion of TSH was recognized. TSHoma is characterized by non-suppressed TSH levels with high levels of free thyroid hormones (fT4 and fT3). Despite their functioning nature, late diagnosis, incidental diagnosis, and misdiagnosis are common.
TSHoma is rare pituitary adenomas with a prevalence of 0.5–2%.[
The age of presentation of TSHoma is variable; most cases are diagnosed in the fifth or sixth decades.[
Except for Cases 5 and 8, all other patients underwent a long diagnostic process and/or treatment before presenting to us. Lack of recognition of the characteristic biochemical abnormality, failure to disclose compressive symptoms, incomplete TFT (TSH only), and poor awareness among general practitioners were the factors contributing to delayed diagnosis. Six patients received prior treatment in the form of thyroxine (n = 3), anti-thyroid drugs (n = 4), and total thyroidectomy (n = 1). The median latency from the beginning of the first symptom to diagnosis was 2.5 years. In four of our cases, the diagnosis was made within 2 years of presentation which could be attributed to referral bias and a lower threshold for cranial imaging.
Presentation of TSHoma can be variable, from asymptomatic to life-threatening apoplexy. Clinical features (in descending order of frequency) include goiter, symptoms of thyrotoxicosis, defects in vision, headache, MPHD, and galactorrhea.[
Pituitary imaging became important in the diagnosis of TSHoma, due to lack of dynamic tests, in the current study. CEMRI sella revealed macroadenoma in all eight cases. In larger series of TSHoma, 15–30% microadenomas have been reported.[
The European thyroid association has advocated surgical resection as the definitive therapy for TSHoma.[
Pituitary radiotherapy and SSAs are two adjunctive treatment modalities for TSHoma, particularly residual disease.[
On tissue examination, TSHoma cells are chromophobic, polymorphic (angular or irregular) and have long cytoplasmic processes with stippled chromatin and prominent nucleoli.[
There are multiple prespecified criteria for remission of TSHoma.[
Our study has certain lacunae. First, various dynamic tests and biological effects that have been described for TSHoma (TRH stimulation, T3 suppression, α-GSU, SHBG, and ICTP) were not performed. Second, postoctreotide and antithyroid drug administration normalization of T4, fT4, GH, and shrinkage in adenoma size were not documented. The impact of various intra-operative findings and post-operative care has not been evaluated.[
CONCLUSION
This is the first study of TSHoma from India. The presence of eight cases of TSHoma among 1500 pituitary surgeries performed over 8 years at a single center, confirms its rarity. In conclusion, this study of TSHoma outlines the heterogeneous clinical presentation (clinically asymptomatic to frank thyrotoxicosis, goiter, and compressive symptoms), biochemical idiosyncrasies (non-suppressed TSH in the presence of elevated free thyroid hormones), treatment dilemmas (TFT not normalized despite erroneous treatment with thyroxine, anti-thyroid drugs or post thyroidectomy), diagnosis in the absence of dynamic tests (biochemical tests and imaging concomitantly), and the frequent requirement of multimodality treatment.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Asioli S, Righi A, Iommi M, Baldovini C, Ambrosi F, Guaraldi F. Validation of a clinicopathological score for the prediction of post-surgical evolution of pituitary adenoma: Retrospective analysis on 566 patients from a tertiary care centre. Eur J Endocrinol. 2019. 180: 127-34
2. Banerjee AK, Sharma BS, Kak VK. Clinically and biochemically silent thyrotroph adenoma with oncocytic change. Neurol India. 2000. 48: 374-7
3. Beck-Peccoz P, Lania A, Beckers A, Chatterjee K, Wemeau JL. 2013, European thyroid association guidelines for the diagnosis and treatment of thyrotropin-secreting pituitary tumors. Eur Thyroid J. 2013. 2: 76-82
4. Beck-Peccoz P, Piscitelli G, Amr S, Ballabio M, Bassetti M, Giannattasio G. Endocrine, biochemical, and morphological studies of a pituitary adenoma secreting growth hormone, thyrotropin (TSH), and alpha-subunit: Evidence for secretion of TSH with increased bioactivity. J Clin Endocrinol Metab. 1986. 62: 704-11
5. Beck-Peccoz P, Persani L, Lania A, De Groot LJ, Chrousos G, Dungan K, Feingold KR, Grossman A, Hershman JM.editors. Thyrotropin-secreting pituitary adenomas. Endotext. South Dartmouth, MA: MDText Inc; 2000. p.
6. Bertholon-Grégoire M, Trouillas J, Guigard MP, Loras B, Tourniaire J. Mono-and plurihormonal thyrotropic pituitary adenomas: Pathological, hormonal and clinical studies in 12 patients. Eur J Endocrinol. 1999. 140: 519-27
7. Brucker-Davis F, Oldfield EH, Skarulis MC, Doppman JL, Weintraub BD. Thyrotropin-secreting pituitary tumors: Diagnostic criteria, thyroid hormone sensitivity, and treatment outcome in 25 patients followed at the national institutes of health. J Clin Endocrinol Metab. 1999. 84: 476-86
8. Dhandapani S, Narayanan R, Jayant SS, Sahoo SK, Dutta P, Walia R. Endonasal endoscopic versus microscopic transsphenoidal surgery in pituitary tumors among the young: A comparative study & meta-analysis. Clin Neurol Neurosurg. 2021. 200: 106411
9. Dutta P, Dhandapani S, Kumar N, Gupta P, Ahuja C, Mukherjee KK. Bevacizumab for radiation induced optic neuritis among aggressive residual/recurrent suprasellar tumors: More than a mere antineoplastic effect. World Neurosurg. 2017. 107: 1044
10. Fang HJ, Fu Y, Wu HW, Sun YL, Li YF, Zhang YZ. Short-term preoperative octreotide for thyrotropin-secreting pituitary adenoma. Chin Med J (Engl). 2017. 130: 936-42
11. Gesundheit N, Petrick PA, Nissim M, Dahlberg PA, Doppman JL, Emerson CH. Thyrotropin-secreting pituitary adenomas: Clinical and biochemical heterogeneity. Case reports and follow-up of nine patients. Ann Intern Med. 1989. 111: 827-35
12. Gupta P, Tripathi M, Dhandapani S, Dutta P. India’s March towards development of treatment for pituitary tumors. Neurol India. 2020. 68: 1183-7
13. Kamoun M, d’Herbomez M, Lemaire C, Fayard A, Desailloud R, Huglo D. Coexistence of thyroid-stimulating hormone-secreting pituitary adenoma and graves’ hyperthyroidism. Eur Thyroid J. 2014. 3: 60-4
14. Kienitz T, Quinkler M, Strasburger CJ, Ventz M. Long-term management in five cases of TSH-secreting pituitary adenomas: A single center study and review of the literature. Eur J Endocrinol. 2007. 157: 39-46
15. Latrech H, Rousseau A, Le Marois E, Billaud L, Bertagna X, Azzoug S. Manifestations and prognosis of thyrotropin-secreting pituitary adenomas: A case series of three patients. Rev Med Interne. 2010. 31: 858-62
16. Losa M, Giovanelli M, Persani L, Mortini P, Faglia G, Beck-Peccoz P. Criteria of cure and follow-up of central hyperthyroidism due to thyrotropin-secreting pituitary adenomas. J Clin Endocrinol Metab. 1996. 81: 3084-90
17. Malchiodi E, Profka E, Ferrante E, Sala E, Verrua E, Campi I. Thyrotropin-secreting pituitary adenomas: Outcome of pituitary surgery and irradiation. J Clin Endocrinol Metab. 2014. 99: 2069-76
18. Marzullo P, Cuocolo A, Ferone D, Pivonello R, Salvatore M, Lombardi G. Cardiac effect of thyrotoxicosis in acromegaly. J Clin Endocrinol Metab. 2000. 85: 1426-32
19. Mete O, Lopes MB. Overview of the 2017 WHO classification of pituitary tumors. Endocr Pathol. 2017. 28: 228-43
20. Mulinda JR, Hasinski S, Rose LI. Successful therapy for a mixed thyrotropin-and prolactin-secreting pituitary macroadenoma with cabergoline. Endocr Pract. 1999. 5: 76-9
21. Negm HM, Al-Mahfoudh R, Pai M, Singh H, Cohen S, Dhandapani S. Reoperative endoscopic endonasal surgery for residual or recurrent pituitary adenomas. J Neurosurg. 2017. 127: 397-408
22. Ónnestam L, Berinder K, Burman P, Dahlqvist P, Engström BE, Wahlberg J. National incidence and prevalence of TSH-secreting pituitary adenomas in Sweden. J Clin Endocrinol Metab. 2013. 98: 626-35
23. Patil NR, Dhandapani S, Sahoo SK, Chhabra R, Singh A, Dutta P.editors. Differential independent impact of the intraoperative use of navigation and angled endoscopes on the surgical outcome of endonasal endoscopy for pituitary tumors: A prospective study. Neurosurg Rev. 2020. p.
24. Rabbiosi S, Peroni E, Tronconi GM, Chiumello G, Losa M, Weber G. Asymptomatic thyrotropin-secreting pituitary macroadenoma in a 13-year-old girl: Successful first-line treatment with somatostatin analogs. Thyroid. 2012. 22: 1076-9
25. Socin HV, Chanson P, Delemer B, Tabarin A, Rohmer V, Mockel J. The changing spectrum of TSH-secreting pituitary adenomas: Diagnosis and management in 43 patients. Eur J Endocrinol. 2003. 148: 433-42
26. Thakur D, Dhandapani M, Ghai S, Mohanty M, Dhandapani S. Intracranial tumors: A nurse-led intervention for educating and supporting patients and their caregivers. Clin J Oncol Nurs. 2019. 23: 315-23
27. Walia R, Gupta R, Bhansali A, Pivonello R, Kumar R, Singh H. Molecular imaging targeting corticotropin-releasing hormone receptor for corticotropinoma: A changing paradigm. J Clin Endocrinol Metab. 2021. 106: e1816-26
28. Webster J, Peters JR, John R, Smith J, Chan V, Hall R. Pituitary stone: Two cases of densely calcified thyrotrophin-secreting pituitary adenomas. Clin Endocrinol (Oxf). 1994. 40: 137-43
29. Yamada S, Fukuhara N, Horiguchi K, Yamaguchi-Okada M, Nishioka H, Takeshita A. Clinicopathological characteristics and therapeutic outcomes in thyrotropin-secreting pituitary adenomas: A single-center study of 90 cases. J Neurosurg. 2014. 121: 1462-73