- Department of Neurosurgery, Center for Research and Training in Neurosurgery, Samaritana University Hospital, Rosario University School of Medicine, Bogotá, Colombia.
Correspondence Address:
David Vergara-Garcia, Derparment of Neurosurgery, Center for Research and Training in Neurosurgery, Samaritana University Hospital, Rosario University School of Medicina, Bogotá, Colombia.
DOI:10.25259/SNI_764_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: David Vergara-Garcia, Juan Felipe Abaunza-Camacho, Mariana Agudelo-Arrieta, William Mauricio Riveros, Alberto Caballero. Transient third cranial nerve palsy after pipeline shield treatment of a ruptured anterior cerebral artery dissecting aneurysm: Case report. 30-Sep-2021;12:489
How to cite this URL: David Vergara-Garcia, Juan Felipe Abaunza-Camacho, Mariana Agudelo-Arrieta, William Mauricio Riveros, Alberto Caballero. Transient third cranial nerve palsy after pipeline shield treatment of a ruptured anterior cerebral artery dissecting aneurysm: Case report. 30-Sep-2021;12:489. Available from: https://surgicalneurologyint.com/surgicalint-articles/11138/
Abstract
Background: Intracranial dissecting aneurysms (IDAs) are rare vascular lesions usually arising from the posterior circulation. The anterior cerebral artery (ACA) is an unusual location for this pathology. Even rarer is the occurrence of a transient de novo third cranial nerve (CN) palsy after flow-diverting device (FDD) treatment of an ACA dissecting aneurysm.
Case Description: A middle-aged man with a prior history of hypertension was admitted to our emergency department with severe headache and loss of consciousness after sexual intercourse. Imaging revealed a subarachnoid hemorrhage with stenosis of the left A1 segment of the ACA. Cerebral digital subtraction angiography confirmed a dissecting aneurysm of the left A1 segment. The aneurysm was treated with an FDD (Pipeline Shield). Transient isolated incomplete third CN palsy was documented 12 h after treatment. No evidence of ischemic or hemorrhagic strokes was found. The condition improved after a few days of empiric steroid treatment.
Conclusion: An FDD is a suitable alternative for the treatment of a ruptured IDA of the anterior circulation. Some infrequent complications associated with the device, such as de novo cranial neuropathies, are yet to be studied.
Keywords: Anterior cerebral artery, Dissecting aneurysm, Flow diversion, Intracranial aneurysm, Oculomotor nerve disease
INTRODUCTION
Intracranial dissecting aneurysms (IDAs) account for 3% of all intracranial aneurysms (IAs) and are usually located in the posterior circulation (PCirc).[
CLINICAL PRESENTATION
History and physical examination
A 49-year-old man with a medical history of hypertension complained of a 3-day history of thunderclap headache after sexual intercourse, followed by vomiting episodes and a generalized tonic-clonic seizure. Besides nuchal rigidity, the physical examination was unremarkable.
Imaging
A computed tomography (CT) of the head revealed a left frontoparietal SAH and a hyperdensity in the proximal segment (A1) of the left ACA [
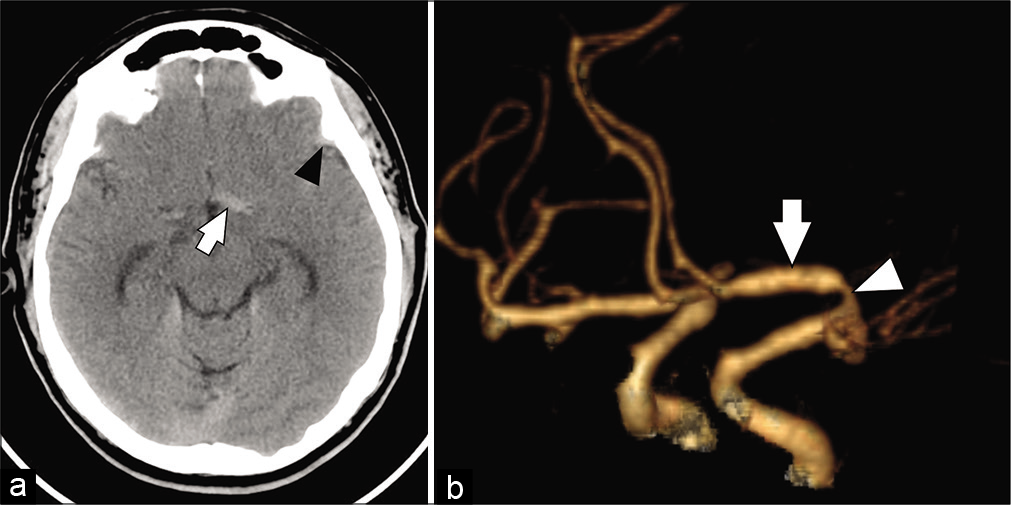
Figure 1:
(a) Axial view of a head computed tomography revealed a left frontoparietal subarachnoid hemorrhage (black arrowhead) and a hyperdensity (white arrow) in the proximal segment (A1) of the left anterior cerebral artery. (b) Oblique view of a head computed tomography angiography with 3D reconstruction showed proximal stenosis (white arrowhead) and post stenotic dilation (white arrow) of the left A1 segment.
Treatment
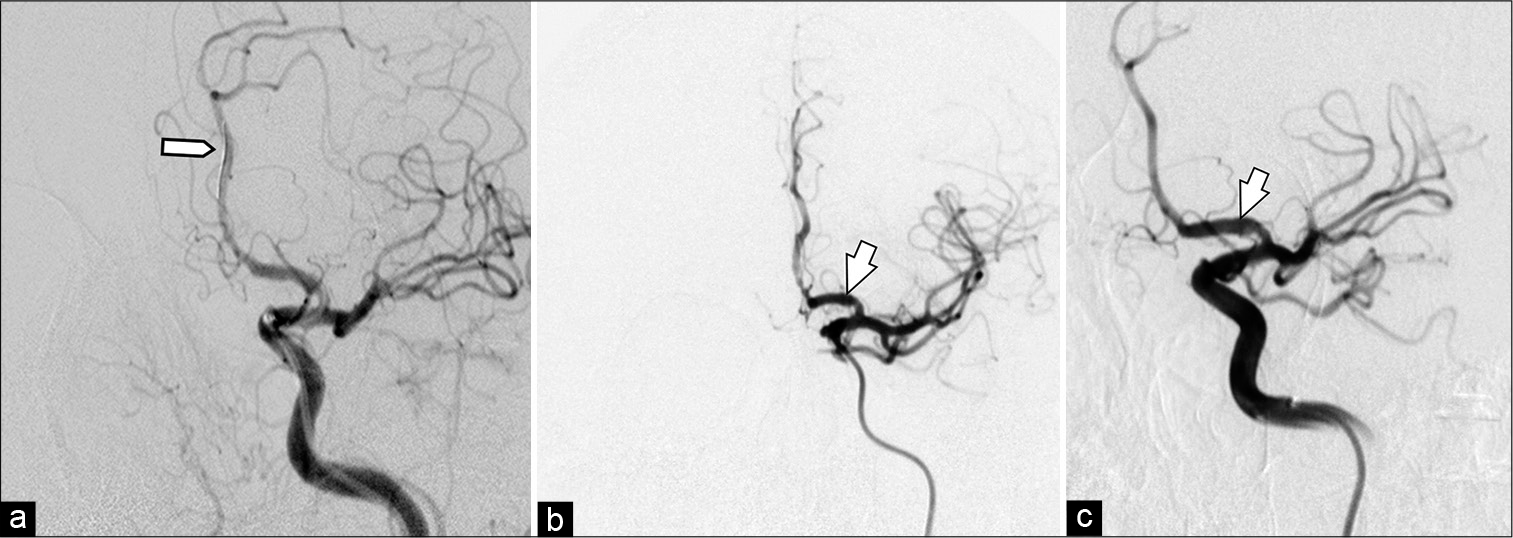
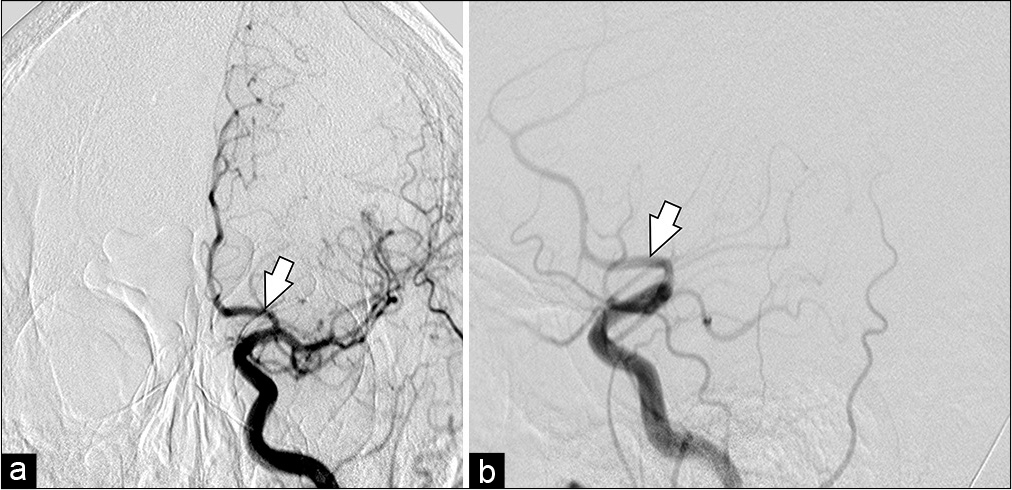
A decision was made to proceed with urgent EVT of the aneurysm. The patient received loading doses of dual antiplatelet therapy (DAPT) for 48 h (acetylsalicylic acid 300 mg/day and clopidogrel 300 mg/day) and then was taken to the neuroangiography suite. After induction of general anesthesia, intraoperative infusion of tirofiban (0.16 mcg/kg/min) was initiated. Before A3 segment microcatheterization with PhenomTM 027 microcatheter (Medtronic Inc., Irvine, California, USA) in Avigo™ Hydrophilic Guidewire (Medtronic Inc., Irvine, California, USA) A Pipeline Flex Embolization Device with Shield Technology (Pipeline Shield; Medtronic Inc., Irvine, California, USA) was strategically deployed, from the A2-segment to A1-segment of the left ACA. Post procedure angiography showed adequate permeability of the FDD [
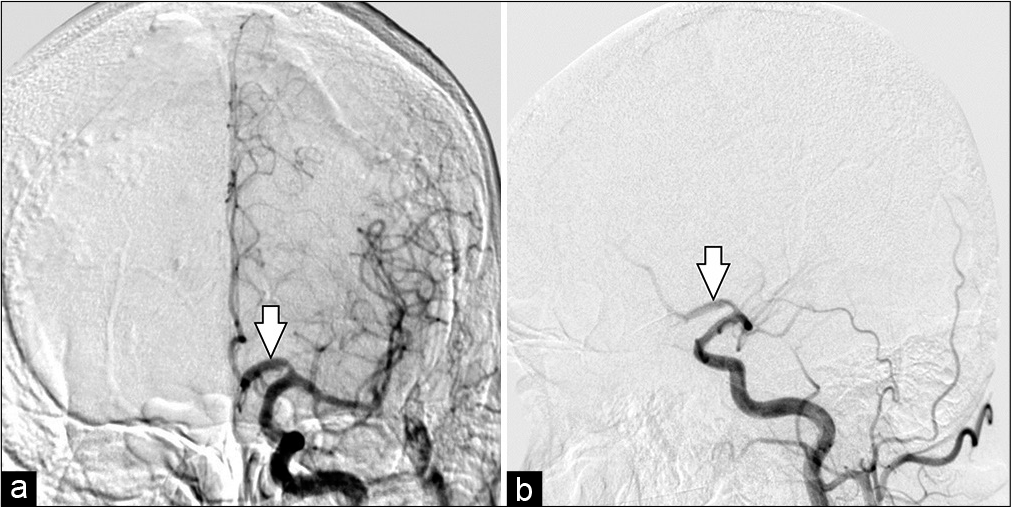
Figure 3:
Digital subtraction angiography anteroposterior projection. Road mapping microcatheterization until A3-segment (a) and PPD implantation from A2 to A1 was performed. Immediate post procedural cerebral digital subtraction angiography. Anteroposterior (b) and oblique (c) views of a left internal carotid artery contrast injection after deployment of the Pipeline shield in the left A1 segment (white arrow).
Post procedural care
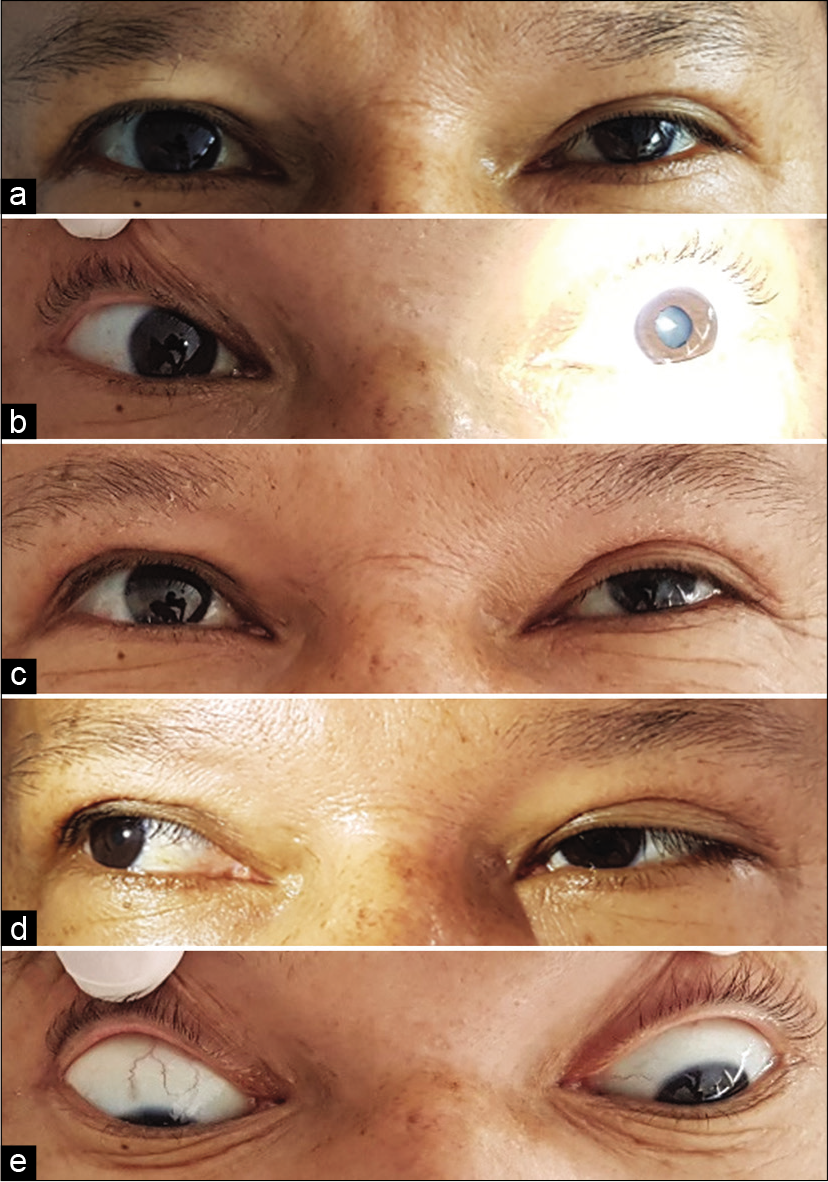
The patient was transferred to the neurointensive care unit. Tirofiban infusion was continued for the next 24 h, and the DAPT was instated to a maintenance dose (acetylsalicylic acid 100 mg/day and clopidogrel 75 mg/day). Twelve hours after the end of the procedure, the patient complained of intermittent binocular diplopia. On examination, there was evidence of preserved visual acuity for both eyes; mild left-eye ptosis; a non-reactive dilated left pupil; left superior, medial, inferior recti, and inferior oblique muscle paresis [
DISCUSSION
IDAs
IDAs account for 3% of all IAs, with a yearly incidence rate of 1–1.5 per 100,000 people.[
Treatment of IDAs
Surgical therapy and EVT have proved to be successful for the treatment of IDAs. However, EVT has become the treatment of choice on account of its high occlusion and lower morbidity and mortality rates.[
Regarding EVT for ruptured IDAs, the evidence is predominantly based on case series of PCirc lesions. Chan et al. conducted a single-center retrospective review of eight patients with IDAs of the PCirc treated with the PED, reporting no major procedure-related complications (hemorrhagic or ischemic complications).[
Third CN Palsy
Third CN palsies are rare in patients with ACA aneurysms, and various etiologic hypotheses have been established for this condition.[
To the best of our knowledge, the patient described in this report is the first documented case of a transient isolated incomplete third CN palsy after successful Pipeline Shield treatment of an ACA IDA without evidence of peri-procedural hemorrhagic or ischemic events. This complication might be a consequence of the inflammatory response generated by the deployment of the FDD or the hemotoxicity exerted by the SAH. However, the precise underlying pathophysiological mechanisms remain uncertain and are yet to be investigated.
CONCLUSION
An FDD efficiently is a suitable alternative for adequately treating a ruptured IDA of the ACirc with high occlusion and lower morbidity and mortality rates especially PED.[
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Ahmad S. Endovascular treatment of ruptured and unruptured intracranial dissecting aneurysms. Interdiscip Neurosurg. 2019. 18: 100510
2. Chan RS, Mak CH, Wong AK, Chan KY, Leung KM. Use of the pipeline embolization device to treat recently ruptured dissecting cerebral aneurysms. Interv Neuroradiol. 2014. 20: 436-41
3. Fairbanks C, White JB. Oculomotor nerve palsy in the setting of an anterior cerebral A2 segment aneurysm. J Neurointerv Surg. 2011. 3: 74-6
4. Hensler J, Jensen-Kondering U, Ulmer S, Jansen O. Spontaneous dissections of the anterior cerebral artery: A meta-analysis of the literature and three recent cases. Neuroradiology. 2016. 58: 997-1004
5. Hofman PA. Dissecting aneurysm of the anterior cerebral artery presenting with thrombo-embolic complications. A case report. Interv Neuroradiol. 2004. 10: 341-6
6. Korkmazer B, Kocak B, Islak C, Kocer N, Kizilkilic O. Long-term results of flow diversion in the treatment of intracranial aneurysms: A retrospective data analysis of a single center. Acta Neurochir (Wien). 2019. 161: 1165-73
7. Lodi YM, Thomas JG, Fessler RD, Ringer AJ.editors. Chapter 35 - Dissecting Intracranial Aneurysms. Intracranial Aneurysms [Internet]. Academic Press; 2018. p. 629-43
8. Martinez-Galdamez M, Lamin SM, Lagios KG, Liebig T, Ciceri EF, Chapot R. Treatment of intracranial aneurysms using the pipeline flex embolization device with shield technology: Angiographic and safety outcomes at 1-year follow-up. J Neurointerv Surg. 2019. 11: 396-9
9. Pasarikovski CR, Waggass G, Cardinell J, Howard P, da Costa L, Yang VX. Pipeline embolisation device with shield technology for the treatment of ruptured intracranial aneurysm. Neuroradiol J. 2019. 32: 189-92
10. Samaniego EA, Gibson E, Nakagawa D, Ortega-Gutierrez S, Zanaty M, Roa JA. Safety of tirofiban and dual antiplatelet therapy in treating intracranial aneurysms. Stroke Vasc Neurol. 2019. 4: 36-42
11. Trivelato FP, Wajnberg E, Rezende MT, Ulhôa AC, Piske RL, Abud TG. Safety and effectiveness of the pipeline flex embolization device with shield technology for the treatment of intracranial aneurysms: Midterm results from a multicenter study. Neurosurgery. 2020. 87: 104-11