- Department of Neurosurgery, Brain Research Institute, Niigata University, Shibata, Niigata, Japan
- Department of Neurosurgery, Niigata Prefectural Shibata Hospital, Shibata, Niigata, Japan.
Correspondence Address:
Daiju Mitsuhashi, Department of Neurosurgery, Brain Research Institute, Niigata University, Niigata, Japan.
DOI:10.25259/SNI_109_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Daiju Mitsuhashi1,2, Takuya Okada1, Keisuke Sato2, Naoki Yajima2, Toyotaka Aiba2. Unique image findings around “kissing” distal anterior cerebral artery aneurysms in addition to perianeurysmal edema: A case report. 26-May-2023;14:181
How to cite this URL: Daiju Mitsuhashi1,2, Takuya Okada1, Keisuke Sato2, Naoki Yajima2, Toyotaka Aiba2. Unique image findings around “kissing” distal anterior cerebral artery aneurysms in addition to perianeurysmal edema: A case report. 26-May-2023;14:181. Available from: https://surgicalneurologyint.com/surgicalint-articles/12338/
Abstract
Background: Some aneurysms cause edema formation in the surrounding brain parenchyma and are thought to reflect various phenomena occurring in the aneurysm. Some authors highlighted perianeurysmal edema (PAE) as a finding that indicates higher risk of rupture of the aneurysm. On the other hand, there are no reports of image changes in the surrounding brain parenchyma of aneurysm other than edema formation.
Case Description: We describe a 63-year-old man with unique signal change in the surrounding brain parenchyma of “kissing” distal anterior cerebral artery aneurysms completely different from PAE. The large and partially thrombosed aneurysm presented well-defined signal change surrounding brain parenchyma in addition to PAE. Intraoperative findings revealed the signal change as a space of retaining serous fluid. Drain the fluid and clipping was made for the both anterior cerebral artery aneurysms. The postoperative course was uneventful and his headache was improved the day after the surgery. The perianeurysmal signal change was also disappeared immediately after the surgery except for the PAE.
Conclusion: This case demonstrates a rare phenomenon of signal change around the aneurysm, and there is a possibility that the unique finding exists as an early manifestation of intracerebral hematoma associated with aneurysm rupture.
Keywords: Clipping, Distal anterior cerebral artery, Kissing aneurysm, Perianeurysmal edema, Thrombosed aneurysm
INTRODUCTION
It has been reported that edematous change can occur in the surrounding brain parenchyma of untreated cerebral aneurysms.[
CASE REPORT
A 63-year-old man with a history of hypertension presented with a moderate headache lasting 1 week. Fifteen years ago, he had a mild headache and had a computed tomography (CT) of the brain at another hospital and it was evaluated as normal. This time, CT of the brain revealed a high-density mass and surrounding well-defined low density area in the left frontal lobe [
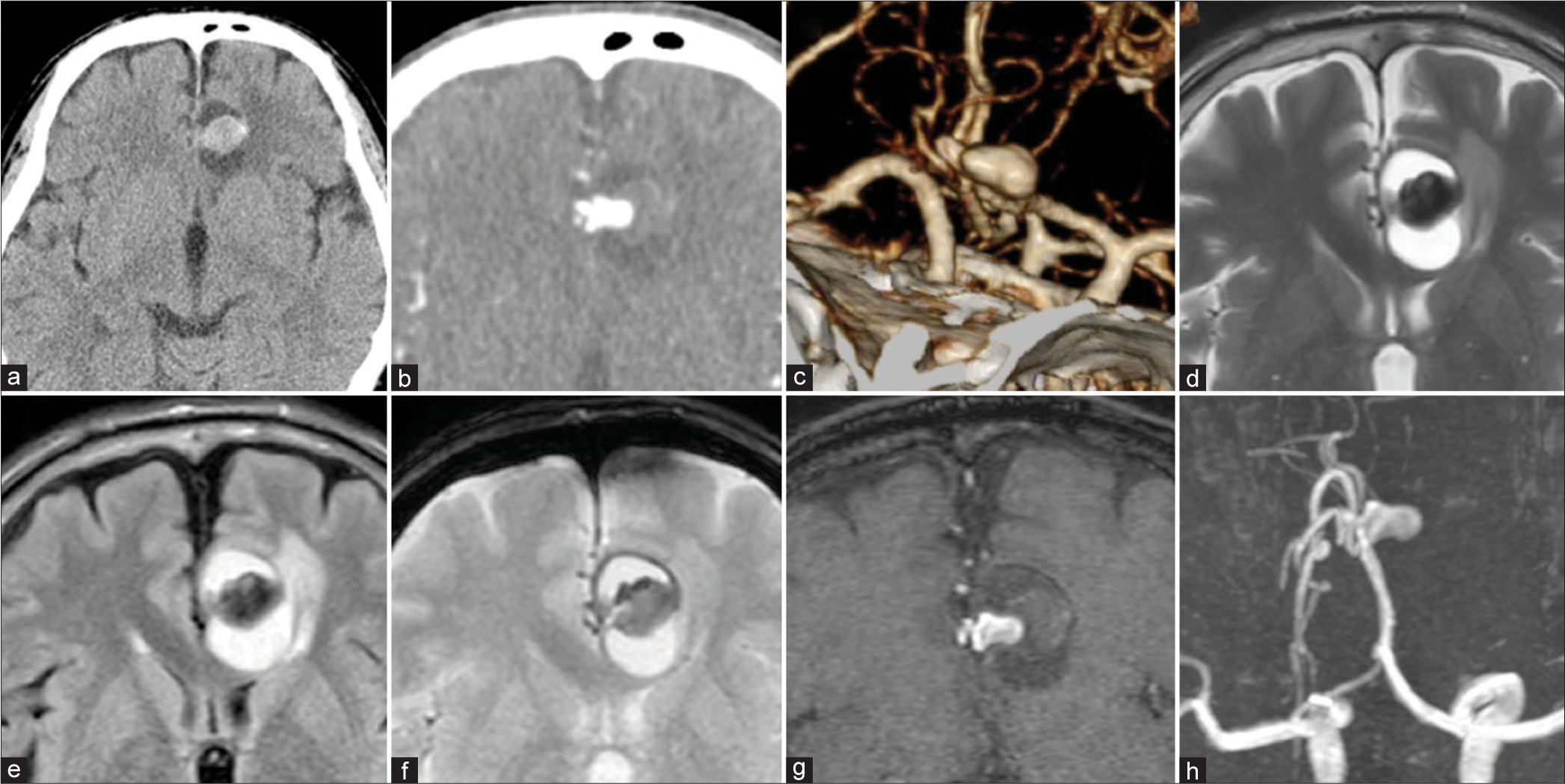
Figure 1:
Preoperative computed tomography (CT) and magnetic resonance imaging. (a) Non-contrast CT, (b) CT angiography, (c) three-dimensional-CT angiography, (d) T2 weighted image, (e) fluid-attenuated inversion recovery image, (f) T2*-weighted image. A part of the aneurysm wall and the front rim of the signal change area present low intensity on T2*. (g) Source image of time-of-flight magnetic resonance angiography, (h) Maximum intensity projection image, Blood flow is observed only in a part of the aneurysm, and there is a free space on the outside showing a thrombus. T2* stands for T2 star-weighted image.
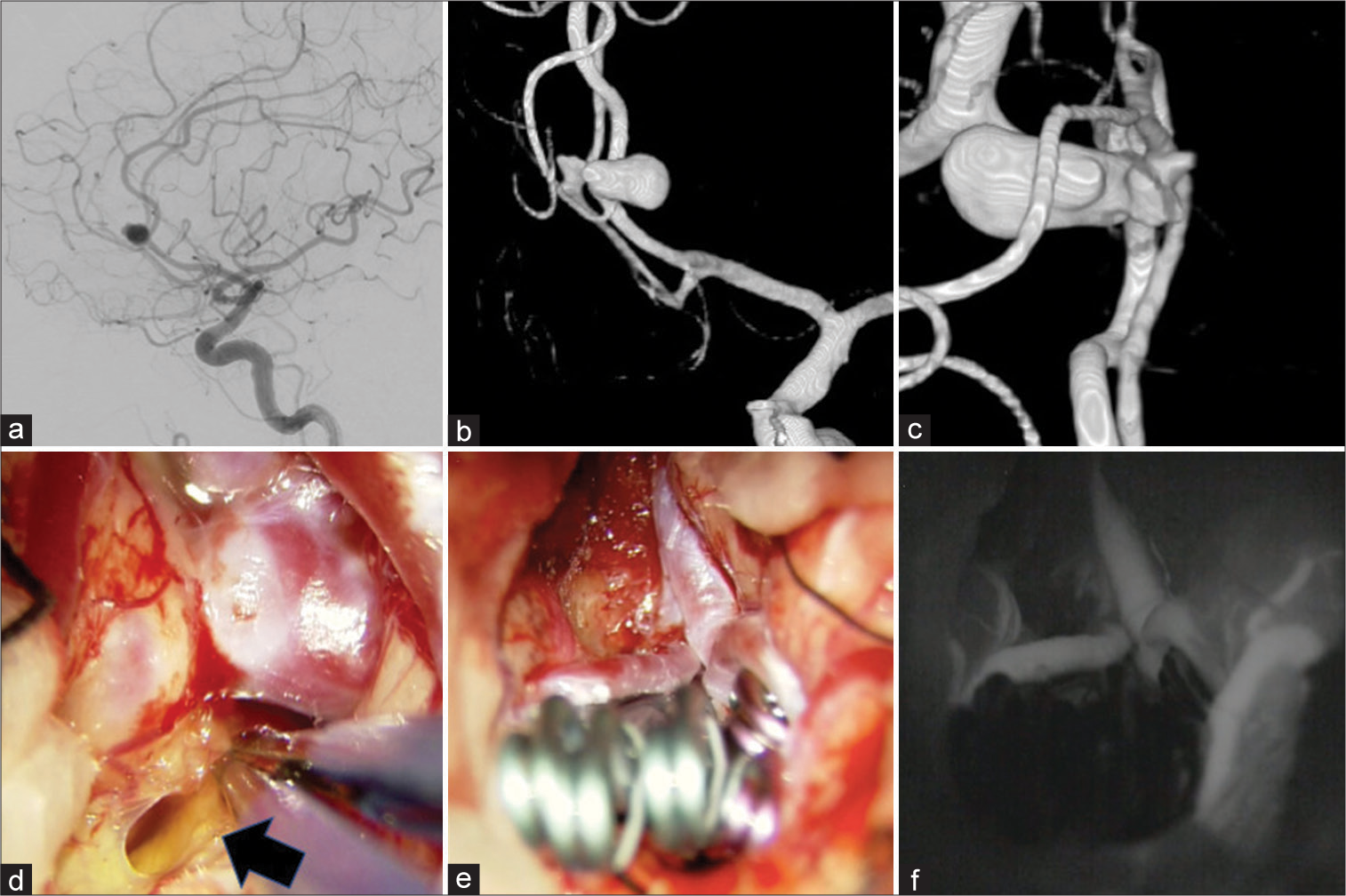
Figure 2:
(a) Lateral view of left internal carotid artery angiogram, (b) three-dimensional digital subtraction angiography (3D-DSA) image, (c) operative view of 3D-DSA image, There is a large aneurysm at the genu portion of the left anterior cerebral artery (ACA), and a multilobular aneurysm at the genu portion of the right ACA. Both aneurysm domes are pressed together. (d-f) Intraoperative photographs during clipping of “kissing” distal ACA aneurysms. (d) Opening the space around the left thrombosed aneurysm, yellowish serous fluid was drained (black arrow). The surrounding brain parenchyma was also yellowish to indicate the presence of inflammation. (e) Multiple clips were needed to obliterate both aneurysms. Because of lack of mobility of the aneurysms and limited horizontal space, application of the clips were restricted and should be inserted along the visual axis of the surgical field. (f) Obliteration of the aneurysms and patency of the parent arteries and the branches were confirmed by venous infusion of the indocyanine green.
Coronal incision and bifrontal craniotomy were made and to dissect the interhemispheric fissure. First, the proximal arteries of bilateral ACA were secured in case of unexpected rupture. Second, an attempt was made to separate the aneurysms, but the complete separation could not be achieved because the aneurysms were tightly adhered and the risk of rupture was assumed to be very high. Most of the left thrombosed aneurysm buried in the frontal lobe and a thin brain parenchyma attached around the neck was incised, a slight yellowish serous fluid was drained [
The postoperative course was uneventful. His headache was improved the day after the surgery, and CT scans revealed disappearance of perianeurysmal signal changes except for the PAE [
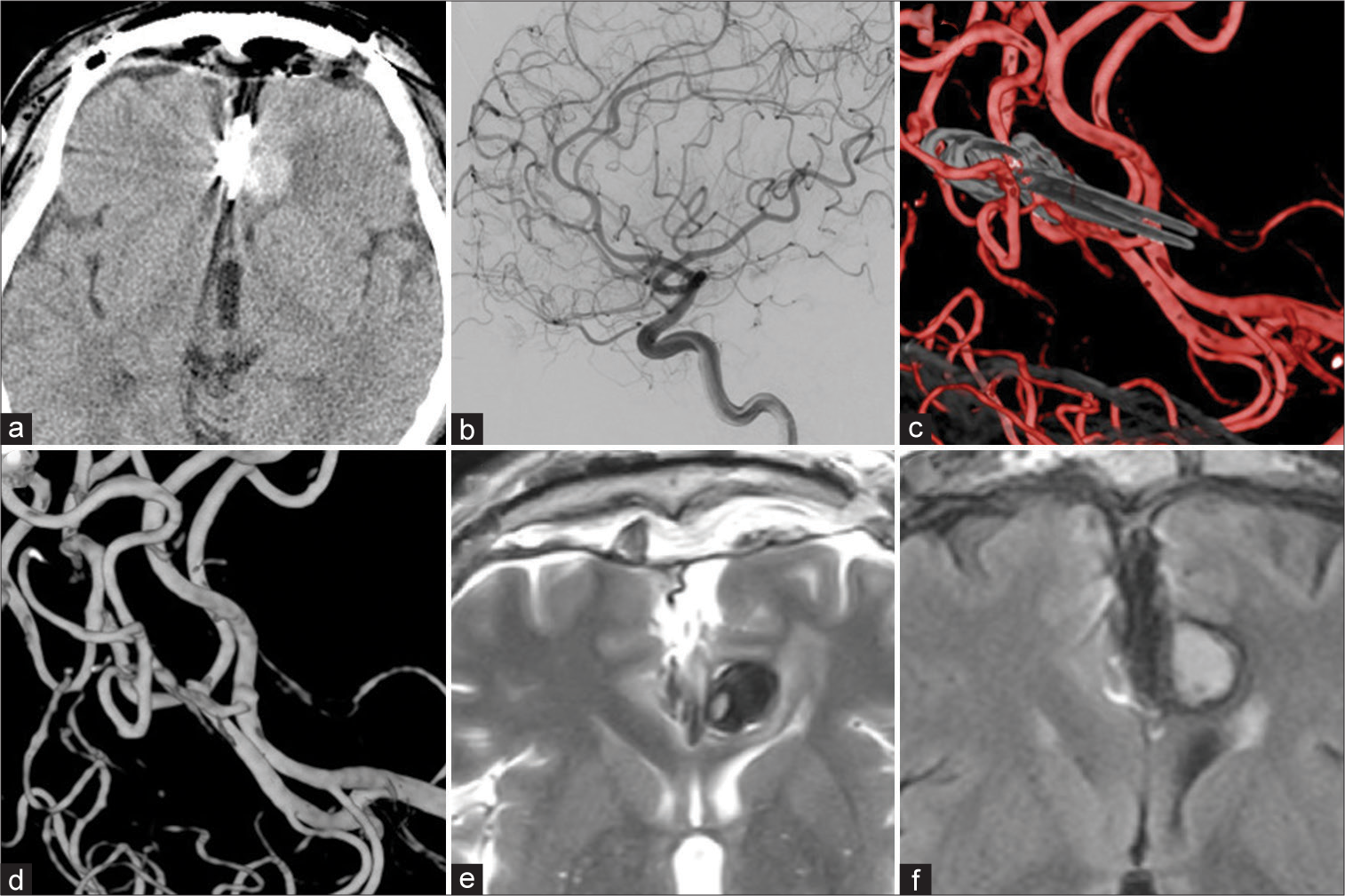
Figure 3:
(a) Non-contrast computed tomography the day after the surgery revealed disappearance of perianeurysmal signal changes except for the perianeurysmal edema (PAE) (b) Lateral view of postoperative left internal carotid artery angiogram, (c and d) Postoperative three-dimensional digital subtraction angiography images, obliteration of the both aneurysms were confirmed. (e) T2-weighted image at 6 months after the operation, (f) fluid-attenuated inversion recovery image at 6 months after the operation, Recurrence of the image change around the aneurysm was not observed and both images showed improvement of the PAE.
DISCUSSION
In this case, quite unique findings different from PAE were observed around the left aneurysm. Both CT and MRI revealed a well-defined cystic lesion which was more prominent than PAE around the aneurysm. To the best of our knowledge, there have been no reports of the signal changes around cerebral aneurysm as in this case.
In our case, a relative acute enlargement is thought to have occurred in the aneurysm. A part of the thrombus in the aneurysm wall showed a lower signal on T2*-weighted image and these suggested the existence of a fresh intra-mural thrombus and repetitive intra-mural bleeding. Thrombosed aneurysms were characterized by recurrent subacute dissections that resulted in repeated intra-mural hemorrhage lead to the progressive enlargement of the aneurysm.[
Some of ruptured aneurysms present intracerebral hematoma (ICH), and in rare cases, present without subarachnoid hemorrhage.[
CONCLUSION
We have described the case of “kissing” DACA aneurysms presented unique findings in addition to PAE. Since DACA is a site that rupture easily associated with ICH, the serous fluid retention around the aneurysm may be a finding that captures the early manifestation of ICH formation. Early treatment should be considered for aneurysms with these surrounding image changes, including PAE.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Abbed KM, Ogilvy CS. Intracerebral hematoma from aneurysm rupture. Neurosurg Focus. 2003. 15: E4
2. Akabane A, Jokura H, Ogasawara K, Takahashi K, Sugai K, Ogawa A. Rapid development of an intranidal aneurysm with perifocal brain edema in an unruptured cerebral arteriovenous malformation. Case report. J Neurosurg. 2002. 97: 1436-40
3. Berge J, Tourdias T, Moreau JF, Barreau X, Dousset V. Perianeurysmal brain inflammation after flow-diversion treatment. AJNR Am J Neuroradiol. 2011. 32: 1930-4
4. Dengler J, Maldaner N, Bijlenga P, Burkhardt JK, Graewe A, Guhl S. Perianeurysmal edema in giant intracranial aneurysms in relation to aneurysm location, size, and partial thrombosis. J Neurosurg. 2015. 123: 446-52
5. Heros RC, Kolluri S. Giant intracranial aneurysms presenting with massive cerebral edema. Neurosurgery. 1984. 15: 572-7
6. Hiu T, Tsutsumi K, Kitagawa N, Hayashi K, Ujifuku K, Yasunaga A. Progressive perianeurysmal edema preceding the rupture of a small basilar artery aneurysm. Clin Neurol Neurosurg. 2009. 111: 216-9
7. Horie N, Kitagawa N, Morikawa M, Tsutsumi K, Kaminogo M, Nagata I. Progressive perianeurysmal edema induced after endovascular coil embolization. Report of three cases and review of the literature. J Neurosurg. 2007. 106: 916-20
8. Jabbari R, Reinhard M, Roelz R, Shah M, Niesen WD, Kaier K. Intracerebral hematoma due to aneurysm rupture: Are there risk factors beyond aneurysm location?. Neurosurgery. 2016. 78: 813-20
9. Krings T, Alvarez H, Reinacher P, Ozanne A, Baccin CE, Gandolfo C. Growth and rupture mechanism of partially thrombosed aneurysms. Interv Neuroradiol. 2007. 13: 117-26
10. Kwan ES, Heilman CB, Shucart WA, Klucznik RP. Enlargement of basilar artery aneurysms following balloon occlusion--“water-hammer effect”. Report of two cases. J Neurosurg. 1991. 75: 963-8
11. Nakayama Y, Tanaka A, Ohshiro S, Yoshinaga S. Extensive edema in the thalamus caused by thrombosed basilar artery aneurysm. Neurol Med Chir (Tokyo). 1998. 38: 274-7
12. Onofrj V, Tampieri D, Cianfoni A, Ventura E. Peri-aneurysmal brain edema in native and treated aneurysms: The role of thrombosis. Neurointervention. 2021. 16: 70-7
13. Pahl FH, de Oliveira MF, Ferreira NP, de Macedo LL, Brock RS, de Souza VC. Perianeurysmal edema as a predictive sign of aneurysmal rupture. J Neurosurg. 2014. 121: 1112-4
14. Schubiger O, Valavanis A, Wichmann W. Growth-mechanism of giant intracranial aneurysms; demonstration by CT and MR imaging. Neuroradiology. 1987. 29: 266-71
15. Thai QA, Raza SM, Pradilla G, Tamargo RJ. Aneurysmal rupture without subarachnoid hemorrhage: Case series and literature review. Neurosurgery. 2005. 57: 225-9
16. Ushikoshi S, Kikuchi Y, Houkin K, Miyasaka K, Abe H. Aggravation of brainstem symptoms caused by a large superior cerebellar artery aneurysm after embolization by Guglielmi detachable coils--case report. Neurol Med Chir (Tokyo). 1999. 39: 524-9