- Department of Neurosurgery, Fuji Brain Institute and Hospital, Fujinomiya, Shizuoka,
- Department of Neurosurgery, NTT Medical Center, Shinagawa,
- Department of Neurosurgery, The University of Tokyo Hospital, Bunkyo, Tokyo, Japan.
Correspondence Address:
Hideaki Ono
Department of Neurosurgery, Fuji Brain Institute and Hospital, Fujinomiya, Shizuoka,
DOI:10.25259/SNI_627_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Yudai Hirano1, Hideaki Ono1, Tomohiro Inoue2, Tomohiro Mitani3, Takeo Tanishima1, Akira Tamura1, Isamu Saito1. Emergent surgical embolectomy for middle cerebral artery occlusion related to cerebral angiography followed by neck clipping for an unruptured aneurysm in the anterior communicating artery. 04-Dec-2020;11:420
How to cite this URL: Yudai Hirano1, Hideaki Ono1, Tomohiro Inoue2, Tomohiro Mitani3, Takeo Tanishima1, Akira Tamura1, Isamu Saito1. Emergent surgical embolectomy for middle cerebral artery occlusion related to cerebral angiography followed by neck clipping for an unruptured aneurysm in the anterior communicating artery. 04-Dec-2020;11:420. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=10425
Abstract
Background: Intracranial embolism related to cerebral angiography is rare but one of the complications of the procedure. However, the standard management of acute intracranial embolism for this etiology has not been established, and there have been very few reports in the past.
Case Description: A 68-year-old male was incidentally found to have an unruptured aneurysm of anterior communicating artery (ACoA). Immediately after the cerebral angiography for the purpose of detailed examination of the aneurysm, the right partial hemiparalysis and mild aphasia developed. Magnetic resonance imaging/angiography (MRI/A) revealed an occlusion in the peripheral part of the left middle cerebral artery (MCA). Due to the existence of magnetic resonance angiography-diffusion mismatch, emergent craniotomy was immediately performed to remove intra-arterial thrombus. We also performed clipping for an unruptured ACoA aneurysm with this approach. Postoperative MRI/A showed that the occluded artery was recanalized and a slight infarction was observed in the left cerebral hemisphere. The patient was discharged on foot and followed at outpatient clinic over 4 years without no neurological deficit.
Conclusion: Emergent surgical embolectomy for distal MCA occlusion related to cerebral angiography followed by neck clipping for an unruptured aneurysm of the ACoA was successful in treating acute occlusion of the peripheral part of the MCA in a patient with an unruptured aneurysm. As there are few similar cases, there is controversy about the best management, but this surgical method can be a safe and effective treatment.
Keywords: Acute ischemic stroke, Cerebral angiography, Embolectomy, Intracranial embolism, Unruptured aneurysm
INTRODUCTION
Recently, new modalities such as three-dimensional (3D) computed tomography angiography (CTA, 3D-CTA) and magnetic resonance imaging/angiography (MRI/A) have emerged instead of digital subtraction angiography (DSA) for diagnostic studies of patients with cerebral aneurysms or intracranial vascular lesions.[
CASE DESCRIPTION
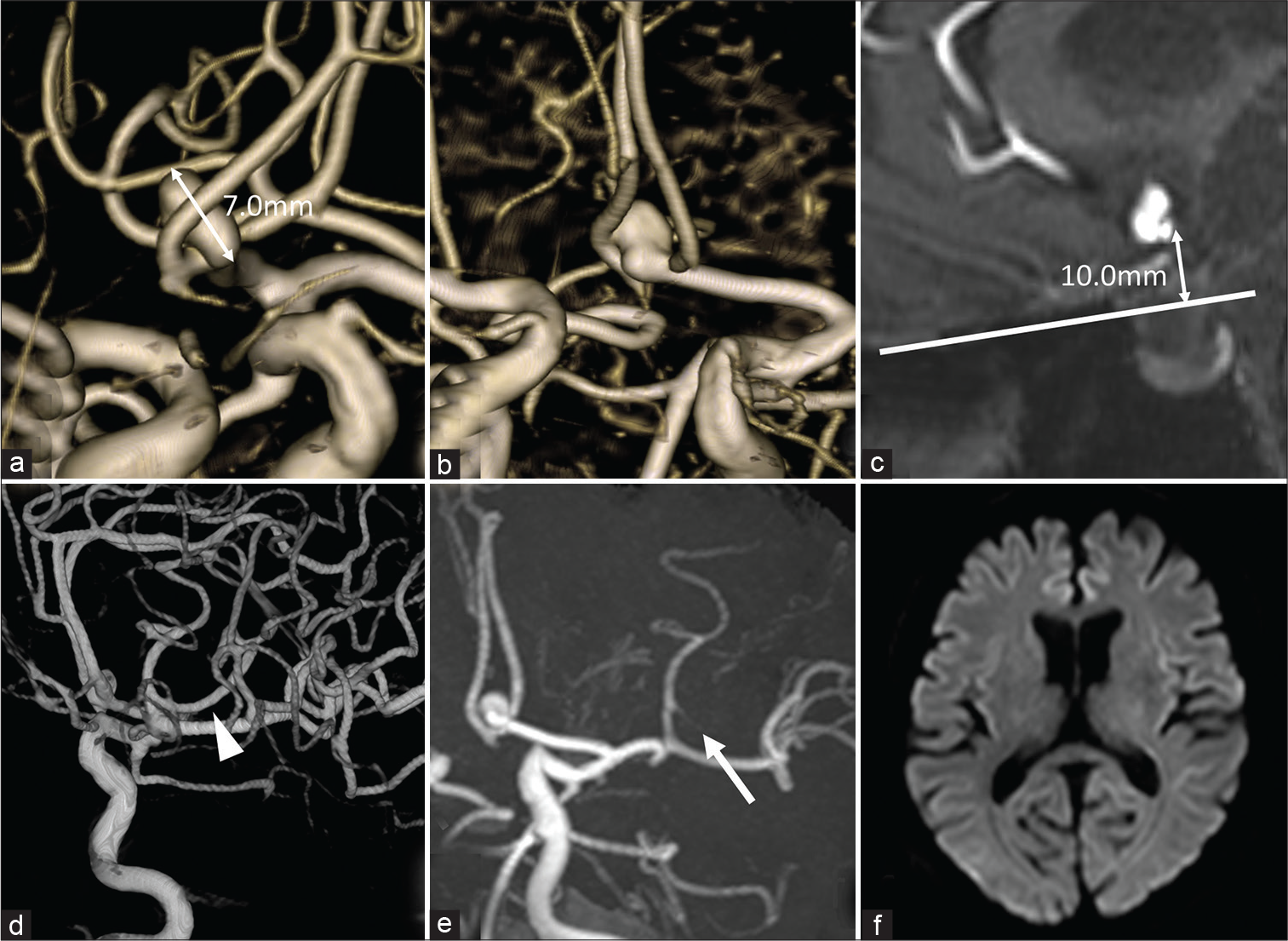
A 68-year-old man with a medical history of hypertension and hyperuricemia was incidentally found to have unruptured aneurysms of the ACoA and right internal carotid artery (ICA) – posterior communicating artery (PCoA) bifurcation. His head computed tomography (CT) and MRI/A showed no abnormal findings except for the aneurysms. The ACoA aneurysm had a maximum diameter of 7 mm [
Figure 1:
Preoperative imaging. (a-c) Magnetic resonance angiography (MRA) showed an unruptured aneurysm of anterior communicating artery, 7 mm in maximum diameter (a), which was protruding upward (b) and located over 10 mm above the frontal base (c). (d and e) M3 portion of middle cerebral artery was occluded in MRA (e, arrow) performed just after the three-dimensional digital subtraction angiography (3D-DSA), which was patent in 3D-DSA (d, arrowhead). (f) No cerebral infarction in diffusion-weighted imaging.
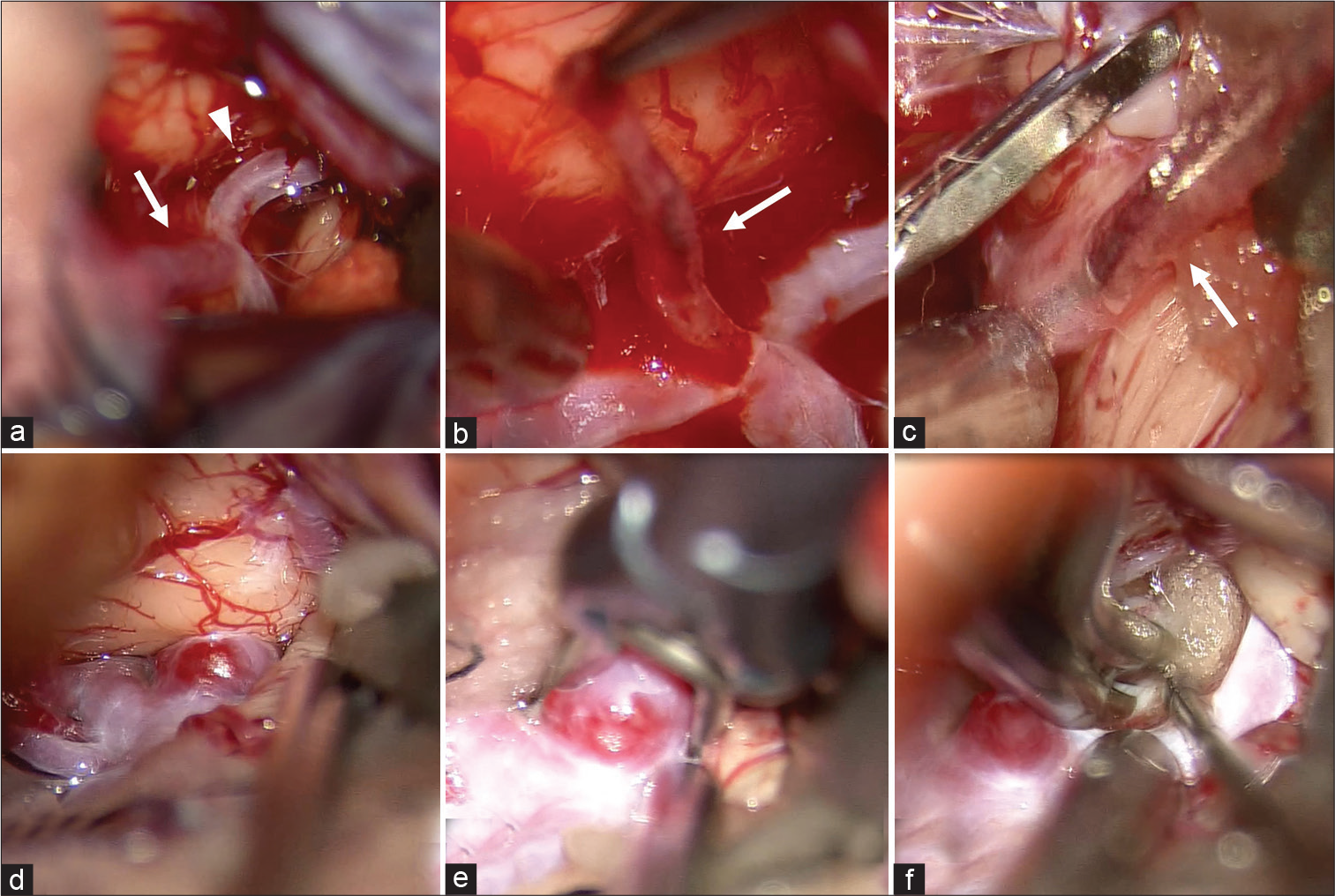
Neuroanesthesia was induced, and the patient was placed in the supine position. The operation was started 80 min after onset. Motor evoked potentials of the right upper limb were monitored. The left frontotemporal craniotomy was performed, and the Sylvian fissure was opened widely from the distal portion under the operating microscope. The M2 superior trunk was identified, and the M2-3 bifurcation was obstructed by an embolus [
Video 1
Figure 2:
Intraoperative photographs. (a) Intra-arterial embolus at M2-3 bifurcation (arrow: M2, arrowhead: M3) of the middle cerebral artery. (b) White color clot (arrow) was removed from the artery and blood flow spouted due to recanalization of occluded vessel. (c) Thrombus (arrow) at the distal M3 was also removed. (d) Anterior communicating artery complex and the aneurysm. (e) Clipping the aneurysm with straight clip. (f) Final view.
Postoperative course
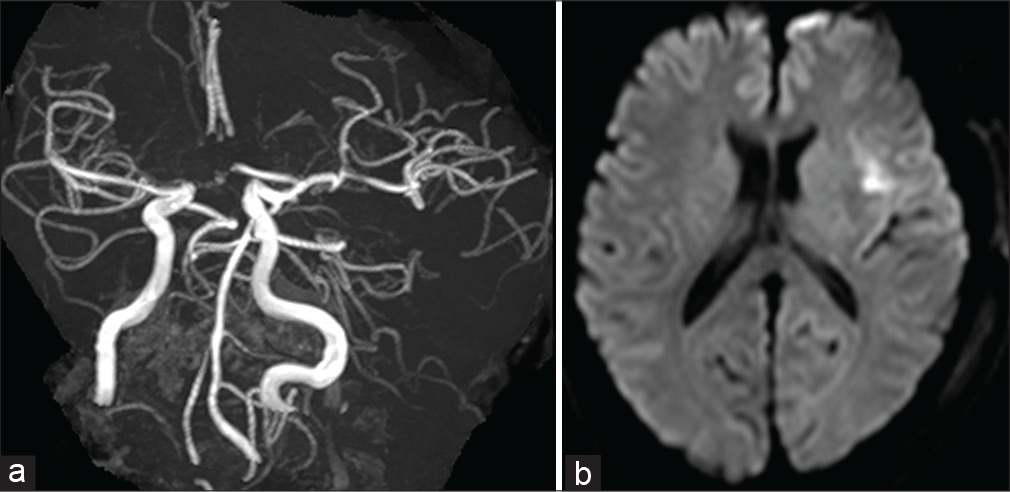
The postoperative course was good. Postoperative MRA revealed recanalization of the occluded MCA and DWI demonstrated a small infarction of the left frontal lobe and insula [
DISCUSSION
Etiology of cerebral infarction related to DSA
DSA is used to examine and treat many types of cerebrovascular diseases.[
DSA may cause various complications, although infrequently. The frequency of neurological complications is about 1–3%, which is decreasing every year, and most of them are transient.[
Multiple mechanisms of neurological complications during DSA have been proposed, which include thrombosis from the catheter and guide wire,[
As far as we know, there have been no previous reports on removing an embolus related to DSA. Macroscopically, the embolus was white and soft; pathologically, it was a fresh thrombus containing a very small amount of red blood cells, without any vascular wall component, and was classified as fibrin-dominant clots.[
Treatment of acute cerebral embolism
The rate of poor outcomes in patients with distal MCA occlusion is reported to be as high as 47.4%, and the rate tends to be higher (64.7%) for a left side occlusion.[
Clipping for the unruptured aneurysm of ACoA
As ACoA aneurysms have a higher rate of rupture than aneurysms in other parts,[
In this case, the left MCA occlusion occurred, and surgical embolectomy was performed. We opened the Sylvian fissure widely from the distal portion identify the occluded M2-3 portion. We also split the interhemispheric fissure widely from the base to identify the neck of the aneurysm, A1, and bilateral A2 without strong retraction of the frontal lobe. As a result, the ACoA complex was clearly confirmed, and clipping was performed safely through the left pterional approach, although the aneurysm was located at a relatively high position.
Limitations
The limitation of this study is that an endovascular team was not available in our hospital or region while treating this case. If an endovascular team had been available, we would have considered endovascular thrombectomy as the first option.
CONCLUSION
Emergent surgical embolectomy for MCA occlusion related to DSA followed by neck clipping for an unruptured aneurysm of the ACoA was successful in treating acute occlusion of the peripheral part of the MCA in a patient with an unruptured aneurysm. As there are few similar cases, there is controversy about the best management, but this surgical method can be a safe and effective treatment.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Videos available on:
www.surgicalneurologyint.com
References
1. Altenbernd J, Kuhnt O, Hennigs S, Hilker R, Loehr C. Frontline ADAPT therapy to treat patients with symptomatic M2 and M3 occlusions in acute ischemic stroke: Initial experience with the penumbra ACE and 3MAX reperfusion system. J Neurointerv Surg. 2018. 10: 434-9
2. Arnold M, Fischer U, Schroth G, Nedeltchev K, Isenegger J, Remonda L. Intra-arterial thrombolysis of acute iatrogenic intracranial arterial occlusion attributable to neuroendovascular procedures or coronary angiography. Stroke. 2008. 39: 1491-5
3. Bendszus M, Koltzenburg M, Burger R, Warmuth-Metz M, Hofmann E, Solymosi L. Silent embolism in diagnostic cerebral angiography and neurointerventional procedures: A prospective study. Lancet. 1999. 354: 1594-7
4. Choudhri O, Schoen M, Mantha A, Feroze A, Ali R, Lawton MT. Increased risk for complications following diagnostic cerebral angiography in older patients: Trends from the nationwide inpatient sample (1999-2009). J Clin Neurosci. 2016. 32: 109-14
5. Cloft HJ, Joseph GJ, Dion JE. Risk of cerebral angiography in patients with subarachnoid hemorrhage, cerebral aneurysm, and arteriovenous malformation: A meta-analysis. Stroke. 1999. 30: 317-20
6. Dion JE, Gates PC, Fox AJ, Barnett HJ, Blom RJ. Clinical events following neuroangiography: A prospective study. Stroke. 1987. 18: 997-1004
7. Gawel M, Burkett M, Rose FC. Platelet activation following cerebral angiography. Acta Neurol Scand. 1980. 61: 240-3
8. Gory B, Lapergue B, Blanc R, Labreuche J, Machaa MB, Duhamel A. Contact aspiration versus stent retriever in patients with acute ischemic stroke with M2 occlusion in the ASTER randomized trial (contact aspiration versus stent retriever for successful revascularization). Stroke. 2018. 49: 461-4
9. Heiserman JE, Dean BL, Hodak JA, Flom RA, Bird CR, Drayer BP. Neurologic complications of cerebral angiography. AJNR Am J Neuroradiol. 1994. 15: 1401-7
10. Hernández-Pérez M, de la Ossa NP, Aleu A, Millán M, Gomis M, Dorado L. Natural history of acute stroke due to occlusion of the middle cerebral artery and intracranial internal carotid artery. J Neuroimaging. 2014. 24: 354-8
11. Hoffman CE, Santillan A, Rotman L, Gobin YP, Souweidane MM. Complications of cerebral angiography in children younger than 3 years of age. J Neurosurg Pediatr. 2014. 13: 414-9
12. Hoh BL, Cheung AC, Rabinov JD, Pryor JC, Carter BS, Ogilvy CS. Results of a prospective protocol of computed tomographic angiography in place of catheter angiography as the only diagnostic and pretreatment planning study for cerebral aneurysms by a combined neurovascular team. Neurosurgery. 2004. 54: 1329-40
13. Horiuchi T, Nitta J, Miyaoka Y, Nagm A, Tsutsumi K, Ito K. Open embolectomy of large vessel occlusion in the endovascular era: Results of a 12-year single-center experience. World Neurosurg. 2017. 102: 65-71
14. Inoue T, Tamura A, Tsutsumi K, Saito I, Saito N. Surgical embolectomy for large vessel occlusion of anterior circulation. Br J Neurosurg. 2013. 27: 783-90
15. Jayaraman MV, Meyers PM, Derdeyn CP, Fraser JF, Hirsch JA, Hussain MS. Reporting standards for angiographic evaluation and endovascular treatment of cerebral arteriovenous malformations. J Neurointerv Surg. 2012. 4: 325-30
16. Kaufmann TJ, Huston J, Mandrekar JN, Schleck CD, Thielen KR, Kallmes DF. Complications of diagnostic cerebral angiography: Evaluation of 19,826 consecutive patients. Radiology. 2007. 243: 812-9
17. Kiyofuji S, Inoue T, Hasegawa H, Tamura A, Saito I. Emergent surgical embolectomy for middle cerebral artery occlusion due to carotid plaque rupture followed by elective carotid endarterectomy. J Neurosurg. 2014. 121: 631-6
18. Laerum F. Injurious effects of contrast media on human vascular endothelium. Invest Radiol. 1985. 20: S98-9
19. Liebeskind DS, Sanossian N, Yong WH, Starkman S, Tsang MP, Moya AL. CT and MRI early vessel signs reflect clot composition in acute stroke. Stroke. 2011. 42: 1237-43
20. Menon BK, Hill MD, Davalos A, Roos YB, Campbell BC, Dippel DW. Efficacy of endovascular thrombectomy in patients with M2 segment middle cerebral artery occlusions: Meta-analysis of data from the HERMES Collaboration. J Neurointerv Surg. 2019. 11: 1065-9
21. Mokin M, Primiani CT, Ren Z, Kan P, Duckworth E, Turner RD. Endovascular treatment of middle cerebral artery M2 occlusion strokes: Clinical and procedural predictors of outcomes. Neurosurgery. 2017. 81: 795-802
22. Ogawa A, Suzuki M, Sakurai Y, Yoshimoto T. Vascular anomalies associated with aneurysms of the anterior communicating artery: Microsurgical observations. J Neurosurg. 1990. 72: 706-9
23. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K. 2018 guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the American heart association/American stroke association. Stroke. 2018. 49: e46-110
24. Ramakrishnan P, Yoo AJ, Rabinov JD, Ogilvy CS, Hirsch JA, Nogueira RG. Intra-arterial eptifibatide in the management of thromboembolism during endovascular treatment of intracranial aneurysms: Case series and a review of the literature. Interv Neurol. 2013. 2: 19-29
25. Sarraj A, Sangha N, Hussain MS, Wisco D, Vora N, Elijovich L. Endovascular therapy for acute ischemic stroke with occlusion of the middle cerebral artery M2 segment. JAMA Neurol. 2016. 73: 1291-6
26. Seners P, Turc G, Maïer B, Mas JL, Oppenheim C, Baron JC. Incidence and predictors of early recanalization after intravenous thrombolysis: A systematic review and meta-analysis. Stroke. 2016. 47: 2409-12
27. Suzuki M, Fujisawa H, Ishihara H, Yoneda H, Kato S, Ogawa A. Side selection of pterional approach for anterior communicating artery aneurysms--surgical anatomy and strategy. Acta Neurochir (Wien). 2008. 150: 31-9
28. UCAS Japan Investigators, Morita A, Kirino T, Hashi K, Aoki N, Fukuhara S. The natural course of unruptured cerebral aneurysms in a Japanese cohort. N Engl J Med. 2012. 366: 2474-82
29. Willinsky RA, Taylor SM, TerBrugge K, Farb RI, Tomlinson G, Montanera W. Neurologic complications of cerebral angiography: Prospective analysis of 2, 899 procedures and review of the literature. Radiology. 2003. 227: 522-8
30. Yasargil MG, Fox JL. The microsurgical approach to intracranial aneurysms. Surg Neurol. 1975. 3: 7-14