- Department of Neurology, Beth Israel Deaconess Medical Center, Boston, Massachusetts, United States.
- Department of Hematology/Oncology, Beth Israel Deaconess Medical Center, Boston, Massachusetts, United States.
- Department of Hematology/Oncology, Massachusetts General Hospital, Boston, Massachusetts, United States.
- Department of Neurosurgery, Beth Israel Deaconess Medical Center, Boston, Massachusetts, United States.
- Department of Neurosurgery, Columbia Presbyterian Medical Center, New York, United States.
- Department of Radiation Oncology, Beth Israel Deaconess Medical Center, United States.
- Department of Radiation Oncology, Massachusetts General Hospital, Boston, Massachusetts, United States.
DOI:10.25259/SNI_595_2020
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Sydni M. Cole1, Sasmit Sarangi1, David Einstein2, Malgorzata McMasters3, Ron Alterman4, Jeffrey Bruce5, Lauren Hertan6, Helen A. Shih7, Eric T. Wong1. Parkinsonism reversed from treatment of pineal non-germinomatous germ cell tumor. 25-May-2021;12:237
How to cite this URL: Sydni M. Cole1, Sasmit Sarangi1, David Einstein2, Malgorzata McMasters3, Ron Alterman4, Jeffrey Bruce5, Lauren Hertan6, Helen A. Shih7, Eric T. Wong1. Parkinsonism reversed from treatment of pineal non-germinomatous germ cell tumor. 25-May-2021;12:237. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=10827
Abstract
Background: Parkinsonism is a rare complication of non-germinomatous germ cell tumors (NGGCTs) arising from the pineal region.
Case Description: We describe a 23-year-old man who presented with Parinaud syndrome, fatigue, and hypersomnia that were caused by a pineal region NGGCT with yolk sac component and an initial α-fetoprotein (AFP) of 1011.0 ng/ml. MRI revealed that the tumor was causing 10 mm of midline shift and compressing the cerebral aqueduct, the left thalamus, and the midbrain. Obstructive hydrocephalus was relieved by ventriculoperitoneal shunting. Six cycles of induction chemotherapy with ifosfamide, carboplatin, and etoposide reduced tumor size and decreased AFP levels in both serum and cerebrospinal fluid. Following the first cycle, the patient developed asymmetric, bilateral Parkinsonism consisting of bradykinesia, bradyphrenia, facial hypomimia, drooling, and dysphagia. Levodopa, amantadine, and methylphenidate were administered and resulted in symptom improvement. Second look neurosurgery revealed residual yolk sac tumor and a second induction regimen of gemcitabine, paclitaxel, and oxaliplatin was administered for rising AFP. The patient eventually received an autologous bone marrow transplant using a regimen of high-dose carboplatin, thiotepa, and etoposide with concomitant colony-stimulating factor and romiplostim support followed by consolidative proton craniospinal radiotherapy. Posttreatment head MRI showed that no evidence of tumor growth and serum AFP was within normal limits. His Parkinsonism eventually resolved and he was weaned off all dopaminergic drugs.
Conclusion: Bilateral Parkinsonism from NGGCT in this patient is probably caused by pressure on nigrostriatal tracts, substantia nigra, or both. The Parkinsonian symptoms can be reversed by aggressive treatment of the tumor and administration of dopaminergic drugs.
Keywords: Germ cell tumor, Parkinsonism, Pineal region tumor
INTRODUCTION
Parkinsonism is a rare complication of germinomas and non-germinatous germ cell tumors (NGGCTs) arising from the pineal region. These pineal region tumors could directly cause this phenomenon by rostral-caudal extension and compression of the substantia nigra pars compacta in the midbrain and projections in the nigrostriatal tract to the dorsal stratum.[
NGGCTs are sub-classified as “secretory” and “nonsecretory” based on the measurable α-fetoprotein (AFP) and β-human chorionic gonadotropin tumor markers in the cerebrospinal fluid, serum, or both.[
CASE PRESENTATION
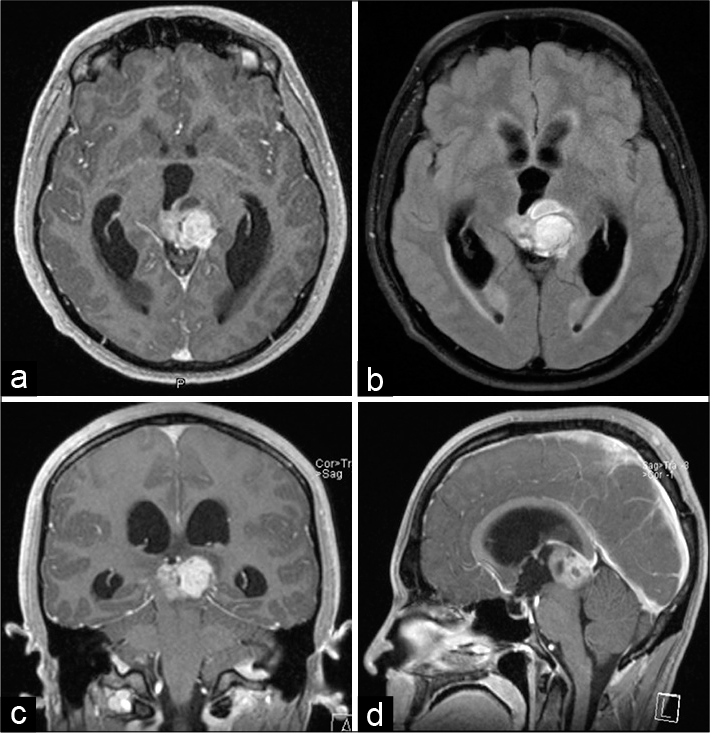
A 23-year-old Caucasian man with history of idiopathic hepatic abscess complicated by portal vein thrombosis developed headache, blurry vision, fatigue, and hypersomnia up to 14 hours of sleep daily. Initial neurologic examination was notable for Parinaud’s syndrome characterized by sluggish pupils, bilateral end-gaze nystagmus, and impaired upgaze. Gadolinium-enhanced head MRI revealed a pineal region mass causing 10 mm of midline shift and compressing the cerebral aqueduct, the left thalamus, and the midbrain [
Figure 1:
Initial presentation of non-germinatous germ cell tumor causing obstructive hydrocephalus. Gadolinium-enhanced T1-weighted head MRI in the axial (a), coronal (c), and sagittal (d) axes performed at presentation showed a pineal region tumor compressing the tectum and causing obstructive hydrocephalus. The FLAIR image in the axial plane (b) revealed some edema in the midbrain.
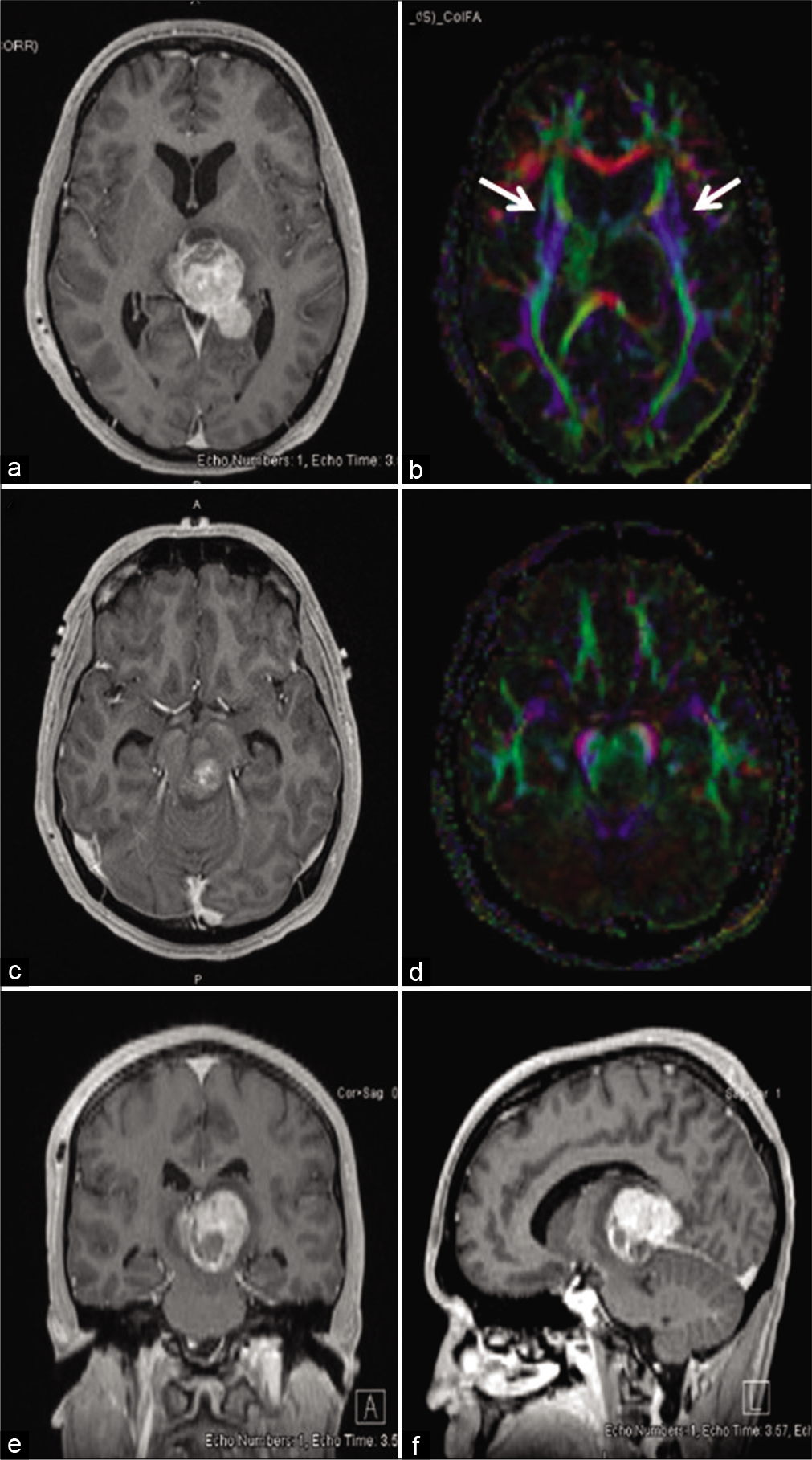
Figure 2:
Rapid growth of non-germinatous germ cell tumor displacing the left substantia nigra and rostral-caudal fibers from midbrain. Gadolinium-enhanced T1-weighted head MRI in the axial (a), coronal (e), and sagittal (f) axes performed before initiation of treatment showed a heterogeneously enhancing mass arising from the pineal region and measuring 34 × 28 × 23 mm. It caused 10 mm of midline shift and extended anteriorly into the left thalamus. The tumor extended caudally into the midbrain on the left exerted pressure medially and anteriorly (c). Diffusion tensor imaging showed that the rostral-caudal fibers (blue color) from the midbrain were displaced anteriorly and laterally on the left and laterally on the right at the pineal (b) and midbrain (d) levels.
The patient’s bilateral Parkinsonism developed after the first dose of induction carboplatin and etoposide, consisting of bradykinesia, bradyphrenia, facial hypomimia, drooling, and dysphagia requiring a gastric tube for feeding. The right side of his body was more severely affected than the left [
Video 1
Video 2
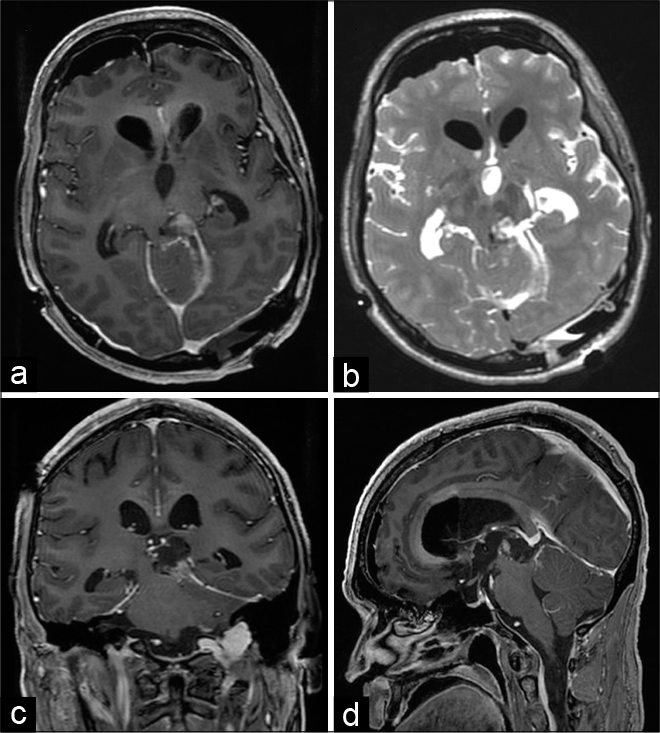
His Parkinsonism improved further after second-look neurosurgery, which achieved near total cytoreduction [
Figure 3:
Second-look neurosurgical resection achieved additional cytoreduction. Gadolinium-enhanced T1-weighted head MRI obtained 1 day after surgery in the axial (a), coronal (c), and sagittal (d) axes, as well as T2-weighted image in the axial plane (b), demonstrated tumor cytoreduction and relief of compression on the tectum and the midbrain.
The patient remains in remission 2 years after transplant. At the time of this case report, the patient is able to perform all activities of daily living. He is able to participate in sporting activities such as jogging [
Video 3
Video 4
DISCUSSION
Central nervous system germ cell tumors are rare, with an incidence of 0.10/100,000 in the United States[
The presenting symptoms of intracranial germ cell tumors are related to their location in the midline near the pineal and suprasellar regions.[
Dysfunction of the left substantia nigra, nigrostriatal tracts, and subsequent decreased dopaminergic input to the respective striatum is most likely the cause of our patient’s bilateral Parkinsonism. It is important to note that his motor manifestations were asymmetric and his right side was more severely affected than the left, consistent with a contralateral (left-sided) nigrostriatal localization. The pretreatment diffusion tensor imaging (DTI) revealed that the rostral-caudal fibers from the midbrain were displaced anteriorly and laterally by the portion of NGGCT located in the left thalamus [
Levodopa treatment improved our patient’s secondary Parkinsonism. Using positron emission tomography, Racette et al.[
Our patient’s Parkinsonism appeared after his first dose of chemotherapy. We suspect that this is related to the cytotoxic effects of the chemotherapeutic agents. Cytotoxic chemotherapy can cause abrupt tumor swelling as cells undergo apoptosis, which may have precipitated the onset of the patient’s Parkinsonism. With ongoing chemotherapy, he began to have an improvement in his Parkinsonism and this improved substantially after second-look surgery. We speculate that in his case, surgery was able to relieve pressure on the substantia nigra and nigrostriatal fibers to a greater extent than chemotherapy. This patient’s younger age and motivation to participate in rehabilitation also aided his recovery, which resulted in his ability to perform all activities of daily living and participate in sports.
There are other case reports that describe Parkinsonism associated with pineal masses.[
CONCLUSION
This case illustrates that aggressive treatments can reverse Parkinsonism from NGGCT, improve disease control, and restore overall quality of life. While poor functional status can be considered as a negative outcome predictor in brain tumors, this should be interpreted in the overall neurological context of the patient and can be potentially reversible.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Videos available on:
www.surgicalneurologyint.com
Acknowledgment
We would like to thank Jonathan L Finlay, MB, ChB, FRCP from the Nationwide Children’s Hospital and The Ohio State University College of Medicine for his expertise and advice.
References
1. Calaminus G, Frappaz D, Kortmann RD, Krefeld B, Saran F, Pietsch T. Outcome of patients with intracranial nongerminomatous germ cell tumors-lessons from the SIOP-CNSGCT-96 trial. Neuro Oncol. 2017. 19: 1661-72
2. Curran T, Lang AE. Parkinsonian syndromes associated with hydrocephalus: Case reports, a review of the literature, and pathophysiological hypotheses. Mov Disord. 1994. 9: 508-20
3. Dolendo MC, Lin TP, Tat OH, Chong QT, Timothy LK. Parkinsonism as an unusual presenting symptom of pineal gland teratoma. Pediatr Neurol. 2003. 28: 310-2
4. Fetcko K, Dey M. Primary central nervous system germ cell tumors: A review and update. Med Res Arch. 2018. 6: 1719
5. Gittleman H, Cioffi G, Vecchione-Koval T, Ostrom QT, Kruchko C, Osorio DS. Descriptive epidemiology of germ cell tumors of the central nervous system diagnosed in the United States from 2006 to 2015. J Neurooncol. 2019. 143: 251-60
6. Goldman S, Bouffet E, Fisher PG, Allen JC, Robertson PL, Chuba PJ. Phase II trial assessing the ability of neoadjuvant chemotherapy with or without second-look surgery to eliminate measurable disease for nongerminomatous germ cell tumors: A children‘s oncology group study. J Clin Oncol. 2015. 33: 2464-71
7. Jennings MT, Gelman R, Hochberg F. Intracranial germ-cell tumors: Natural history and pathogenesis. J Neurosurg. 1985. 63: 155-67
8. Lo AC, Hodgson D, Dang J, Tyldesley S, Bouffet E, Bartels U. Intracranial germ cell tumors in adolescents and young adults: A 40-year multi-institutional review of outcomes. Int J Radiat Oncol Biol Phys. 2020. 106: 269-78
9. McCarthy BJ, Shibui S, Kayama T, Miyaoka E, Narita Y, Murakami M. Primary CNS germ cell tumors in Japan and the United States: An analysis of 4 tumor registries. Neuro Oncol. 2012. 14: 1194-200
10. Mokhtech M, Rotondo RL, Bradley JA, Sandler ES, Nanda R, Logie N. Early outcomes and patterns of failure following proton therapy for nonmetastatic intracranial nongerminomatous germ cell tumors. Pediatr Blood Cancer. 2018. 65: e26997
11. Racette BA, Esper GJ, Antenor J, Black KJ, Burkey A, Moerlein SM. Pathophysiology of parkinsonism due to hydrocephalus. J Neurol Neurosurg Psychiatry. 2004. 75: 1617-9
12. Vhora S, Kobayashi S, Okudera H. Pineal cavernous angioma presenting with Parkinsonism. J Clin Neurosci. 2001. 8: 263-6
13. Wakai S, Nakamura K, Niizaki K, Nagai M, Nishizawa T, Yokoyama S. Meningioma of the anterior third ventricle presenting with parkinsonism. Surg Neurol. 1984. 21: 88-92
14. Zhang Y, Wu IW, Buckley S, Coffey CS, Foster E, Mendick S. Diffusion tensor imaging of the nigrostriatal fibers in Parkinson‘s disease. Mov Disord. 2015. 30: 1229-36