- Department of Neurosurgery, Itoigawa General Hospital, Itoigawa, Niigata, Japan.
Correspondence Address:
Masahito Katsuki, Department of Neurosurgery, Itoigawa General Hospital, Itoigawa, Niigata, Japan.
DOI:10.25259/SNI_455_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Masahito Katsuki, Shin Kawamura, Akihito Koh. Japanese herbal Kampo medicine, Keishibukuryogan, for chronic subdural hematoma – Prospective observational study. 15-Jul-2022;13:307
How to cite this URL: Masahito Katsuki, Shin Kawamura, Akihito Koh. Japanese herbal Kampo medicine, Keishibukuryogan, for chronic subdural hematoma – Prospective observational study. 15-Jul-2022;13:307. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=11717
Abstract
Background: Pharmacological treatment for chronic subdural hematoma (CSDH) recurrence prevention after surgery is under debate. CSDH may be related to suidoku (fluid disturbance) from the Japanese herbal kampo perspective. Goreisan (GRS) treats suidoku and is used to prevent a postoperative recurrence. However, not all CSDHs are liquid, and some have structures such as trabecula, hematoma, and clots, suggesting oketsu (blood stasis). Therefore, we prospectively investigated the keishibukuryogan (KBG) effectiveness, which treats oketsu, for CSDH recurrence prevention and hematoma resolution compared to GRS.
Methods: We prospectively prescribed KBG 7.5 g/day for 12 CSDH patients after burr-hole surgery. As a control cohort, we retrospectively collected 48 patients treated by GRS 7.5 g/day. The recurrence within 1 month and the hematoma thickness after 1 month were evaluated.
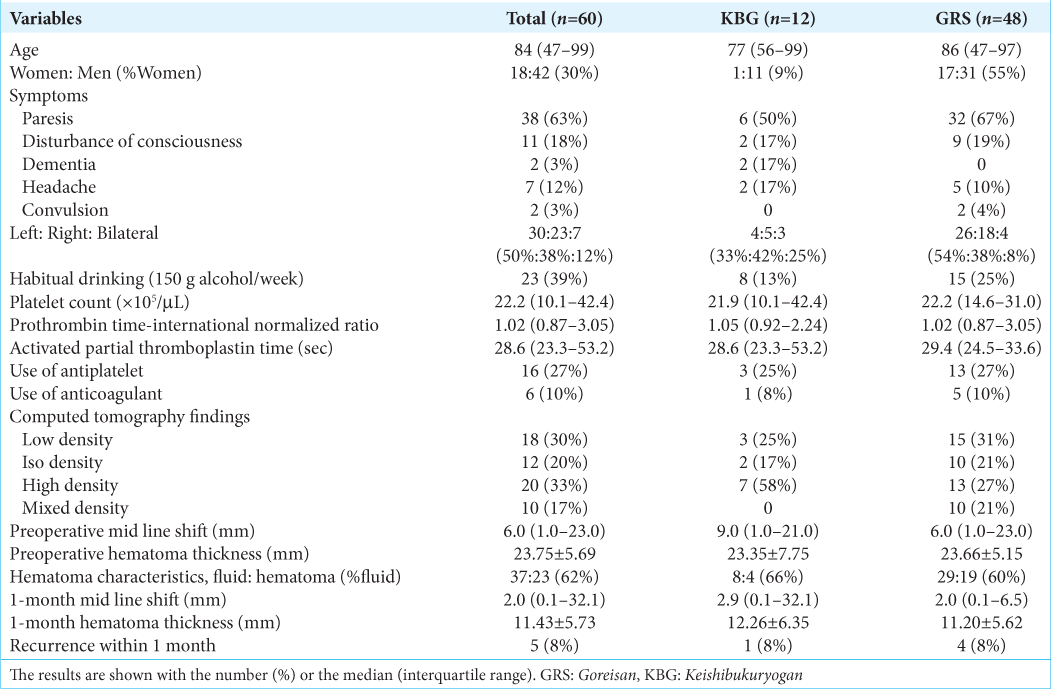
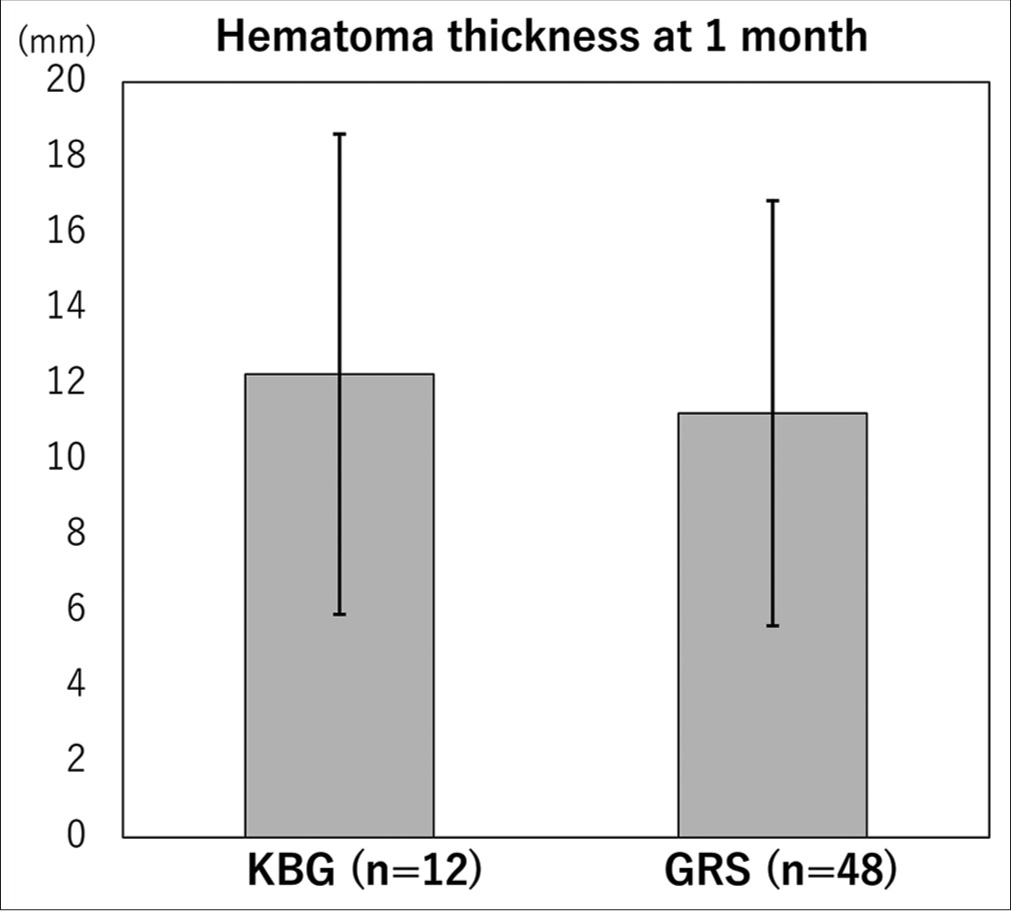
Results: The median age was 84 years old. All the patients’ symptoms improved after surgery. The median preoperative midline shift and mean hematoma thicknesses were 6.0 mm and 23.75 mm. Those at 1 month were 2.0 mm and 11.43 mm. The recurrence rate was not significantly different between the KBG cohort (1 of 12) and the GRS cohort (4 of 48) (P = 0.999). The KBG’s noninferiority to GRS regarding the hematoma thickness at 1 month was statistically proven; KBG (12.26 mm) and GRS (11.20 mm).
Conclusion: The recurrence rate at 1 month was not different between the KBG and GRS cohorts. The hematoma thickness at 1 month in the KBG cohort was not statistically inferior to that in the GRS cohort.
Keywords: Chronic subdural hematoma, Goreisan, Japanese herbal kampo medicine, Keishibukuryogan, Recurrence
INTRODUCTION
Chronic subdural hematoma (CSDH) is common among the aged population, with 13.1–20.6/100,000 individuals in the general population in Japan.[
In Japan, Japanese herbal kampo, goreisan (GRS), is often used postoperatively to prevent CSDH recurrence[
However, not all CSDHs are necessarily liquid, and some are separated or have structures such as trabecula, hematoma, and clots.[
We hypothesized that CSDH can be regarded as a disease related to suidoku and oketsu status and that KBG has a noninferior effect to that of GRS for postoperative CSDH resolution. Therefore, we prospectively prescribed KBG as postoperative medication for recurrence prevention and facilitating postoperative CSDH resolution and compared the efficacy of KBG and GRS.
MATERIALS AND METHODS
Study population and general management
From April 2021 to May 2022, we prospectively collected 12 consecutive CSDH patients who underwent burr-hole surgery under local anesthesia. The CSDH diagnosis was based on the clinical history and the presence of CSDH on computed tomography (CT) and its compression against the brain. Surgical intervention was performed when hematoma thickness was sufficient to compress the brain and when the neurological symptoms were obvious and due to the CSDH. In patients with bilateral hematomas, the operation was performed on both sides. However, when the hematoma on one side was much larger than that on the other side, and the neurological symptoms seemed to be due to the more massive hematoma, we performed burr-hole surgery unilaterally on the responsible side.
We performed single burr-hole surgery with irrigation using artificial cerebrospinal fluid and postoperative drainage under local anesthesia with sedation for all the patients. We made a burr hole on the part of the cranium, where the hematoma was the thickest. After irrigating the hematoma cavity, we inserted the drainage tube toward the frontal cranium. The drainage was removed within 2 days after the operation. All 12 patients took the KBG 7.5 g/day postoperatively at least for a month. Antithrombotic drugs were not ceased during the perioperative period.
As a control cohort against the KBG cohort, we also retrospectively collected 48 consecutive CSDH patients treated by GRS in our hospital from April 2017 to March 2021. The surgical procedure and perioperative management were the same in those treated with KBG. The 48 patients took GRS 7.5 g/day postoperatively at least for a month.
Each patient provided written informed consent for their inclusion in this study, which was approved by the hospital’s research ethics committee (approval number 2021–7). This prospective study was performed following the Declaration of Helsinki.
Clinical variables and outcomes
We collected data regarding physiological symptoms and medical history on admission; age, sex, symptoms, laterality of the hematoma, presence of habitual drinking (150 g alcohol/week), platelet count, prothrombin time-international normalized ratio, activated partial thromboplastin time, the use of antithrombotic drugs, and the recurrence. We also investigated the hematoma density,[
As outcomes, the recurrence within 1 month and the hematoma thickness after 1 month were evaluated. No patients recurred their CSDH after 1 month in the two cohorts.
Statistical analysis
Results were presented as median (interquartile range) for the variables without normal distribution and mean ± standard deviation for the variables with normal distribution. The Shapiro–Wilk test confirmed the normal distribution.
Levene’s test confirmed the equal variance. We compared the KBG and GRS cohorts regarding recurrence rate within 1 month using Fisher’s exact test. We also investigated the KBG’s noninferiority to the GRS regarding the recurrence rate and the hematoma thickness at 1 month. We calculated the sample size needed to prove the KBG’s noninferiority to GRS, using the true recurrence ratio and mean hematoma thickness difference between the KBG cohort and the GRS cohort, its mean standard deviation of the two cohorts, alpha error (0.05), and beta error (0.20). The margins were set as 10% regarding the recurrence rate and 5 mm regarding the hematoma thickness at 1 month. We conducted these analyses using version 28.0.0 of SPSS software (IBM, NY, USA). A one-sided P < 0.05 was considered statistically significant.
RESULTS
General characteristics
Statistical analysis of the recurrence rate and the hematoma thickness at 1 month
The recurrence rate was not significantly different between the KBG cohort (1 of 12, 8%) and the GRS cohort (4 of 48, 8%) (P = 0.999 by Fisher’s exact test). The noninferiority of KBG regarding recurrence rate to GRS could not be proven, because the needed sample number was 99 individuals in each cohort.
The mean hematoma thickness at 1 month was not significantly different between the KBG cohort (12.26 ± 6.35) and the GRS cohort (11.20 ± 5.62) (P = 0.613 by t-test). The noninferiority of KBG regarding recurrence rate to GRS was proven, because the needed sample number was 12 individuals in each cohort (the true difference was 1.06 mm and the mean standard deviation was 5.73 mm) [
DISCUSSION
We, herein, suggest the equivalent efficacy of KBG for postoperative CSDH resolution compared to GRS. The recurrence rate at 1 month was not different between KBG and GRS cohorts. The hematoma thickness at 1 month in KBG cohorts was not inferior to that in GRS cohorts. Our results suggest the possibility of KBG as postoperative medication for recurrence prevention comparable to GRS.
Medication for CSDH
CSDH is hypothesized to be induced by trauma in dural border cells. The sustained inflammation around dural border cells in CSDH results in outer membrane growth and fluid accumulation. This is one of the most convincing hypotheses regarding the pathogenesis of CSDH. Especially, the outer membrane, which contains layers of fibroblasts and collagen fibers with inflammatory cells, is considered important for driving CSDH growth.[
From this context of CSDH pathogenesis, several medications have been used as preventive medicine for CSDH recurrence. Atorvastatin, dexamethasone, and tranexamic acid[
GRS is often used after burr-hole surgery in Japan, expecting its hydrogogue effect to solve suidoku status. Katayama et al.[
Therefore, from the pathophysiology of CSDH, such as inflammation, microbleeds, and coagulopathy, we consider CSDH not only suidoku but also oketsu status from the kampo perspective. Of course, CSDH cannot be considered as only oketsu status-related diseases, but our result of noninferiority of KBG to GRS suggests its possibility. Further studies on the classification of CSDH from the kampo perspective are needed.
Oketsu status and KBG
Previously, two reports on oketsu status and KBG treatment regarding neurosurgical diseases have been reported. Osawa et al.[
KBG ameliorates endothelial function induced by oxidative stress, coagulopathy, hyperviscosity from anti-platelet aggregation, lipid metabolism, regulation of systemic leptin level and lipid metabolism, inflammatory factors, macrophage infiltration, hyperplasia, tissue fibrosis, and sclerosis caused by TGF-β1 and fibronectin, dysfunction of regulated cell deaths, and ovarian hormone imbalance. Clinically, KBG is often used for deep venous thrombosis, rheumatoid arthritis, atopic dermatitis, endometriosis, and hot flash.[
Limitation
Although statistical noninferiority was proven about hematoma thickness at 1 month, our study’s sample size was small. We hoped to show the noninferiority of recurrence rate between KBG and GRS cohorts, but the needed sample number was 100 in each cohort. Furthermore, the hematoma type, such as fluid, and solid, with some structures, should be investigated to consider, whether the CSDH is related to suidoku or oketsu status. Finally, we did not collect the species of the outer membrane for pathological analysis. Besides, we did not perform the tongue diagnosis which is very useful to diagnose oketsu from the kampo perspective.[
CONCLUSION
The recurrence rate at 1 month was not different between KBG and GRS cohorts. In addition, the hematoma thickness at 1 month in KBG cohorts was not inferior to that in GRS cohorts.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Di Cristofori A, Remida P, Patassini M, Piergallini L, Buonanno R, Bruno R. Middle meningeal artery embolization for chronic subdural hematomas. A systematic review of the literature focused on indications, technical aspects, and future possible perspectives. Surg Neurol Int. 2022. 13: 94
2. Fujii S, Kiyokawa J, Tamada N, Yoshimura M, Hirota S, Yamamoto S. Administration of keishibukuryogan in combination with sennoside may accelerate absorption of intracerebral hematomas: Evaluation of six cases (Japanese). J Neurosurg Kampo Med. 2017. 3: 41-7
3. Fujisawa N, Oya S, Yoshida S, Tsuchiya T, Nakamura T, Indo M. A prospective randomized study on the preventive effect of Japanese Herbal Kampo medicine goreisan for recurrence of chronic subdural hematoma. Neurol Med Chir (Tokyo). 2021. 61: 12-20
4. Karibe H, Kameyama M, Kawase M, Hirano T, Kawaguchi T, Tominaga T. Epidemiology of chronic subdural hematomas. No Shinkei Geka. 2011. 39: 1149-53
5. Katayama K, Matsuda N, Kakuta K, Naraoka M, Takemura A, Hasegawa S. The effect of Goreisan on the prevention of chronic subdural hematoma recurrence: Multi-center randomized controlled study. J Neurotrauma. 2018. 35: 1537-42
6. Katsuki M, Kakizawa Y, Wada N, Yamamoto Y, Uchiyama T, Nakamura T. Endoscopically observed outer membrane color of chronic subdural hematoma and histopathological staging: White as a risk factor for recurrence. Neurol Med Chir (Tokyo). 2020. 60: 126-35
7. Katsuki M, Kawamura S, Kashiwagi K, Koh A. Medication overuse headache successfully treated by three types of Japanese Herbal Kampo medicine. Cureus. 2021. 13: e16800
8. Katsuki M, Narita N, Matsumori Y, Ishida N, Watanabe O, Cai S. Kampo (Japanese herbal) medicine for primary headache as an acute treatment-a retrospective investigation in Kesennuma City Hospital during five years. J Neurosurg Kampo Med. 2022. 7:
9. Katsuki M, Yasuda I, Narita N, Ozaki D, Sato Y, Kato Y. Chronic subdural hematoma in patients over 65 years old: Results of using a postoperative cognitive evaluation to determine whether to permit return to driving. Surg Neurol Int. 2021. 12: 212
10. Lanksch W, Kazner E, Grumme T.editors. Computed Tomography in Head Injuries. Berlin, Heidelberg: Springer; 1979. p.
11. Lee KS. History of chronic subdural hematoma. Korean J Neurotrauma. 2015. 11: 27-34
12. Mori K, Maeda M. Surgical treatment of chronic subdural hematoma in 500 consecutive cases: Clinical characteristics, surgical outcome, complications, and recurrence rate. Neurol Med Chir (Tokyo). 2001. 41: 371-81
13. Morita A, Murakami A, Noguchi K, Watanabe Y, Nakaguchi T, Ochi S. Combination image analysis of tongue color and sublingual vein improves the diagnostic accuracy of Oketsu (Blood Stasis) in Kampo Medicine. Front Med (Lausanne). 2021. 8: 790542
14. Nagahori T, Nishijima M, Takaku A. Histological study of the outer membrane of chronic subdural hematoma: Possible mechanism for expansion of hematoma cavity. Neurol Surg. 1993. 21: 697-701
15. Nakaguchi H, Tanishima T, Yoshimasu N. Relationship between drainage catheter location and postoperative recurrence of chronic subdural hematoma after burr-hole irrigation and closed-system drainage. J Neurosurg. 2000. 93: 791-5
16. Osawa SI, Endo H, Kawamura T, Tominaga T. Safety and efficacy of Keishi-Bukuryo-Gan in patients with spontaneous intracerebral hemorrhage during the acute period: CT image-based analysis of the clearance of hematoma. No Shinkei Geka. 2018. 46: 763-70
17. Tanaka K, Chiba K, Nara K. A review on the mechanism and application of keishibukuryogan. Front Nutr. 2021. 8: 760918
18. Terasawa K. Evidence-based reconstruction of kampo medicine: Part II-The concept of sho. Evid Based Complement Alternat Med. 2004. 1: 119-23
19. Wang X, Song J, He Q, You C. Pharmacological treatment in the management of chronic subdural hematoma. Front Aging Neurosci. 2021. 13: 684501
20. Yamada T, Natori Y. Prospective study on the efficacy of orally administered tranexamic acid and Goreisan for the prevention of recurrence after chronic subdural hematoma burr hole surgery. World Neurosurg. 2020. 134: e549-53
21. Yu W, Chen W, Jiang Y, Ma M, Zhang W, Zhang X. Effectiveness comparisons of drug therapy on chronic subdural hematoma recurrence: A Bayesian network meta-analysis and systematic review. Front Pharmacol. 2022. 13: 845386