- Department of Neurosurgery, University of Pittsburgh, Pittsburgh, United States,
- Department of Neurosurgery, University of Baghdad, College of Medicine, Baghdad, Iraq,
- Department of Neurosurgery, University of Cincinnati, Cincinnati, United States,
- Department of Neurosurgery, Al-Nahrain University, Baghdad, Iraq
- Department of Neurosurgery, University of Baghdad, Baghdad, Iraq.
Correspondence Address:
Samer S. Hoz, Department of Neurosurgery, University of Pittsburgh, Pittsburgh, United States.
DOI:10.25259/SNI_58_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Samer S. Hoz1, Mustafa Ismail2, Paolo Palmisciano3, Younus M. Al-Khazaali4, Saleh A. Saleh5, Ahmed Muthana2, Jonathan A. Forbes3, Charles J. Prestigiacomo3, Mario Zuccarello3, Norberto Andaluz3. Cortical incisions and transcortical approaches for intra-axial and intraventricular lesions: A scoping review. 08-Mar-2024;15:82
How to cite this URL: Samer S. Hoz1, Mustafa Ismail2, Paolo Palmisciano3, Younus M. Al-Khazaali4, Saleh A. Saleh5, Ahmed Muthana2, Jonathan A. Forbes3, Charles J. Prestigiacomo3, Mario Zuccarello3, Norberto Andaluz3. Cortical incisions and transcortical approaches for intra-axial and intraventricular lesions: A scoping review. 08-Mar-2024;15:82. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=12785
Abstract
Background: Transcortical approaches, encompassing various surgical corridors, have been employed to treat an array of intraparenchymal or intraventricular brain pathologies, including tumors, vascular malformations, infections, intracerebral hematomas, and epileptic surgery. Designing cortical incisions relies on the lesion location and characteristics, knowledge of eloquent functional anatomy, and advanced imaging such as tractography. Despite their widespread use in neurosurgery, there is a noticeable lack of systematic studies examining their common lobe access points, associated complications, and prevalent pathologies. This scoping review assesses current evidence to guide the selection of transcortical approaches for treating a variety of intracranial pathologies.
Methods: A scoping review was conducted using the PRISMA-ScR guidelines, searching PubMed, EMBASE, Scopus, and Web of Science. Studies were included if ≥5 patients operated on using transcortical approaches, with reported data on clinical features, treatments, and outcomes. Data analysis and synthesis were performed.
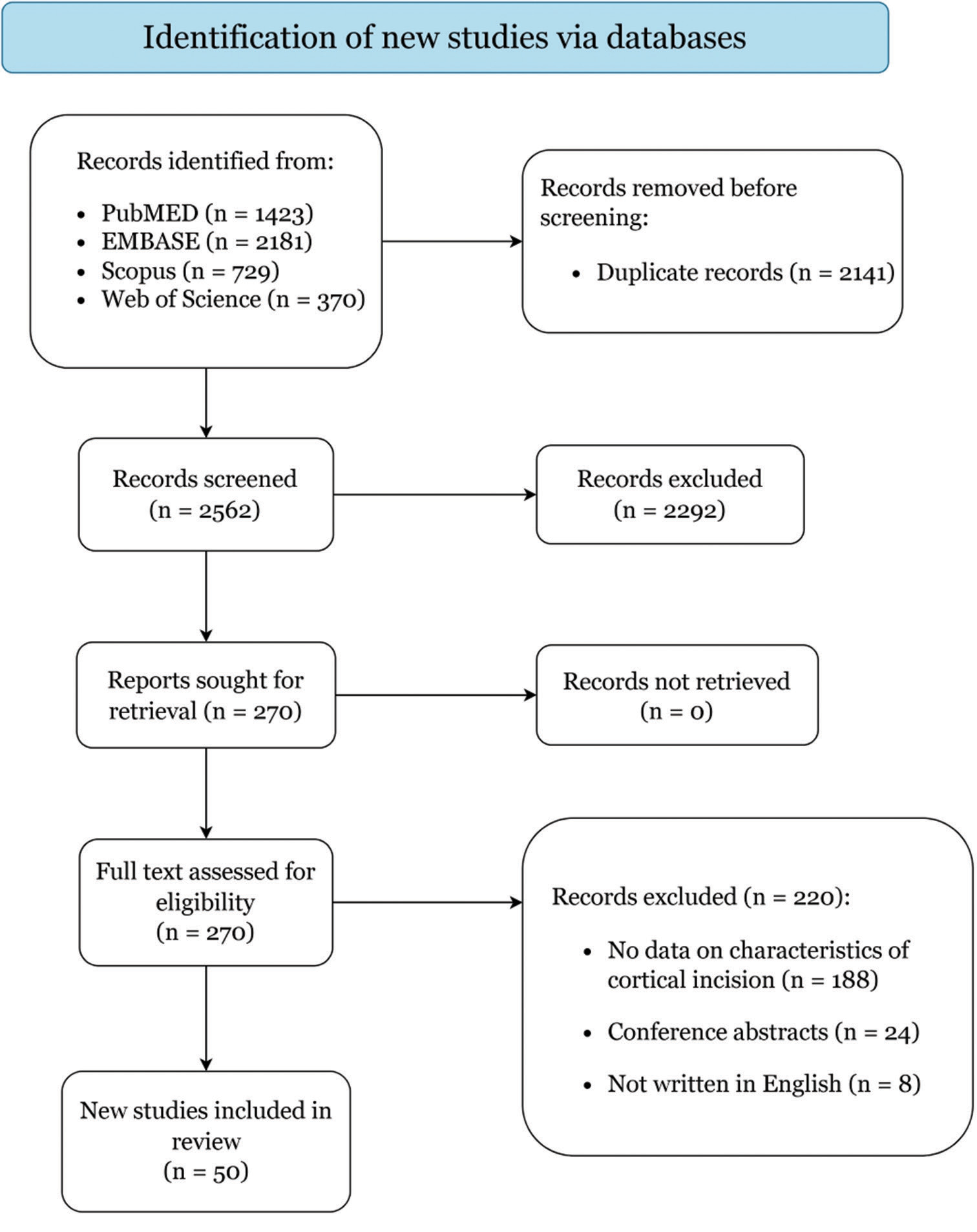
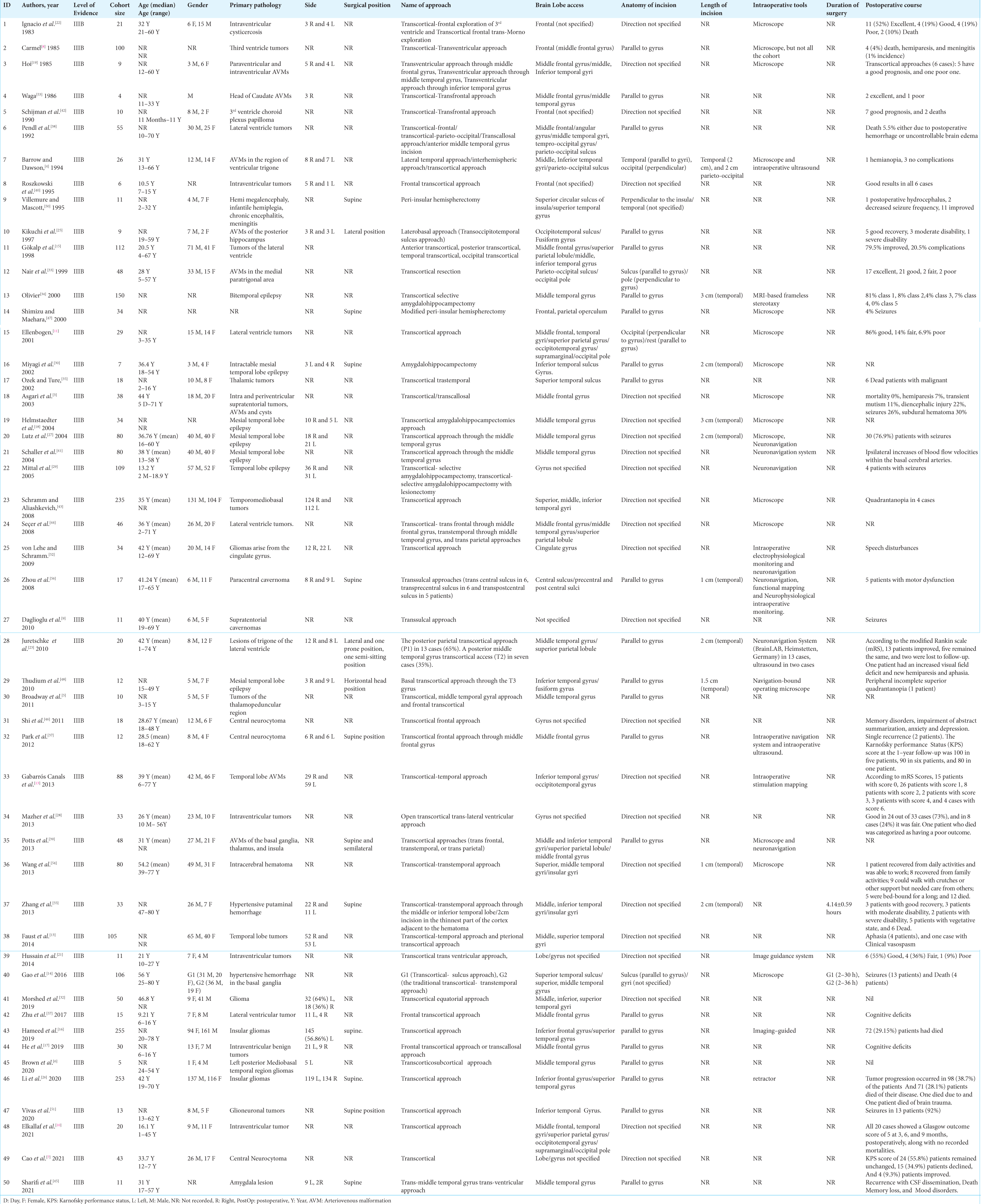
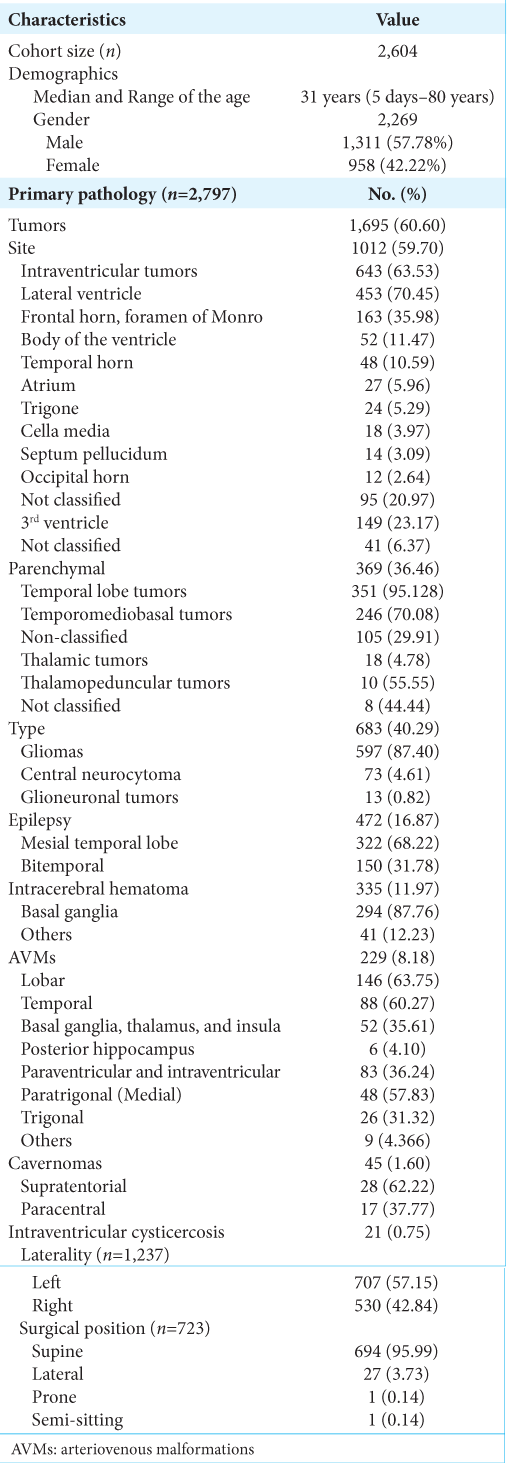
Results: A total of 50 articles encompassing 2604 patients were included in the study. The most common primary pathology was brain tumors (60.6%), particularly gliomas (87.4%). The transcortical-transtemporal approach was the most frequently identified cortical approach (70.48%), and the temporal lobe was the most accessed brain lobe (55.68%). The postoperative course outcomes were reported as good (55.52%), poor (28.38%), and death (14.62%).
Conclusion: Transcortical approaches are crucial techniques for managing a wide range of intracranial lesions, with the transcortical-transtemporal approach being the most common. According to the current literature, the selective choice of cortical incision and surgical corridor based on the lesion’s pathology and anatomic-functional location correlates with acceptable functional outcomes.
Keywords: Intracranial lesions, Surgical approach, Transcortical approaches, Transcortical transtemporal
INTRODUCTION
Transcortical approaches, which involve a wide variety of surgical corridors, have long been utilized to address a diverse range of cranial pathologies.[
Despite the widespread use of transcortical approaches in the field of neurosurgery, there remains a notable scarcity of systematic studies examining their common lobe access points, associated complications, and prevalent pathologies. This scoping review endeavors to fill this knowledge gap by analyzing the available evidence, offering neurosurgeons effective guidance while selecting the most appropriate transcortical approaches for managing a broad spectrum of intracranial lesions.
MATERIALS AND METHODS
Literature search
A scoping review was performed using the PRISMA-ScR guidelines.[
Study selection
The inclusion and exclusion criteria were pre-determinedly set. Studies were included that (1) involved ≥5 patients who were operated on using the transcortical approaches, as explicitly mentioned by the authors; (2) reported parameters on clinical presentation, anatomy-based operative approaches, and outcomes; (3) and were written in English. Studies were excluded if they were as follows: (1) reviews, conference abstracts, animal studies, cadaveric studies, or autopsy reports; (2) studies with an unclear distinction between patients using transcortical approach or not; and (3) studies lacking data on ≥2 of clinical characteristics, approaches, and/or outcomes. In the case of studies with overlapping cohorts, only those with the longest follow-up period were included in the study.
Two independent reviewers (M.I. and A.M.) evaluated the titles and abstracts of all obtained articles before assessing the complete texts of those that met the inclusion criteria. A third reviewer (P.P.) arbitrated any conflicts. Articles that met the inclusion criteria were added, and references were examined to identify additional relevant studies.
Data extraction
Data were extracted by two reviewers (M.I. and A.M.) and confirmed by one additional reviewer (P.P.). The authors did not report missing data. Extracted data included author and year of the study, level of evidence, cohort size, age, gender, primary pathology, side, surgical position, nomenclature of the approach, brain lobe access, anatomy of incision, length of incision, intraoperative tools, duration of the surgery, and postoperative course.
Data synthesis, quality assessment, and statistical analysis
Primary outcomes of interest were outcomes in selected patients who underwent surgery with cortical incision approaches. Two independent reviewers (M.I. and A.M.) assessed the level of evidence for each publication based on the 2011 Oxford Centre For Evidence-Based Medicine recommendations and the risk of bias based on the Joanna Briggs Institute checklists for case reports and case series.[
Continuous variables are summarized as medians and ranges, and categorical variables as frequencies and percentages.
RESULTS
Study selection
Demographics and clinical characteristics
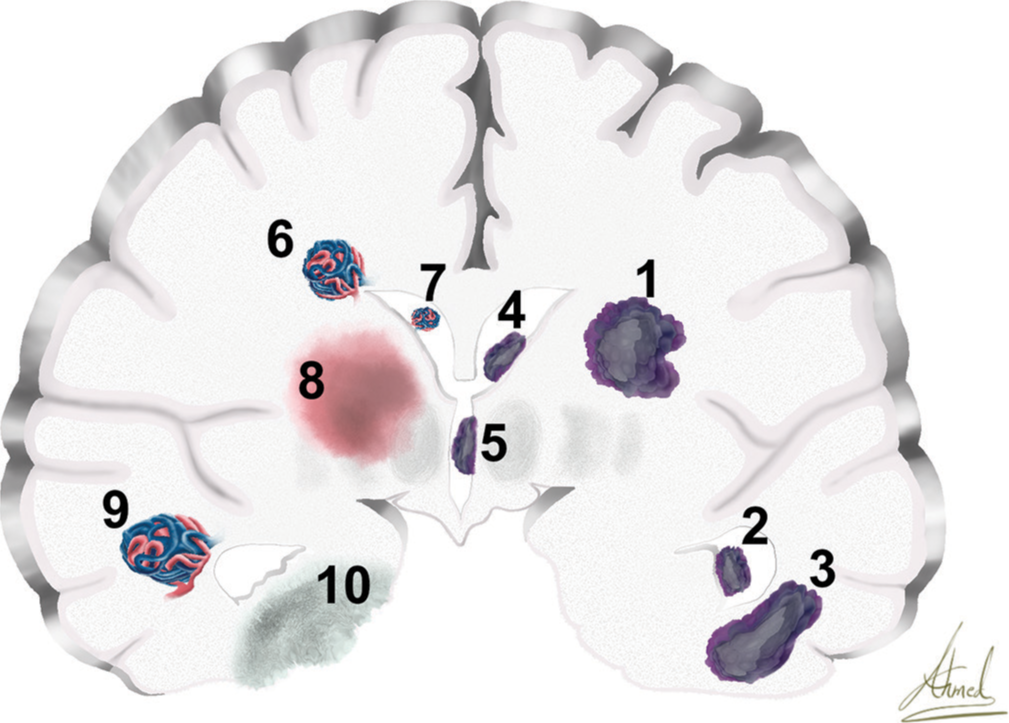
Other pathologies include epilepsy (16.87%), intracerebral hematoma (11.97%), and brain AVMs (8.12%). Most lobar AVMs (63.75% of all AVM cases) were in the temporal area (60.27%), followed by the basal ganglia, thalamus, and insula (35.61%). Furthermore, paraventricular and intraventricular AVMs (36.24% of all AVM cases) were observed, with peritrigonal (medial) AVMs representing 57.83% of cases and trigonal AVMs making up 31.32% of cases. Cavernomas were treated in 1.6% of cases, with 62.22% of them being supratentorial. Intraventricular cysticercosis was found in 0.75% of cases.
Figure 2:
Coronal section of the cerebrum shows different pathologies. (1) glioma, (2) temporal horn of lateral ventricular tumor, (3) temporal lobe tumor, (4) frontal horn of lateral ventricular tumor, (5) third ventricular tumor, (6) paraventricular arteriovenous malformations (AVM), (7) intraventricular AVM, (8) thalamic intracerebral hemorrhage, (9) temporal lobe AVM, and (10) mesial temporal epilepsy.
Management strategies
The surgical position of the patients was mostly supine (95.99%). Treatment strategies are reported in
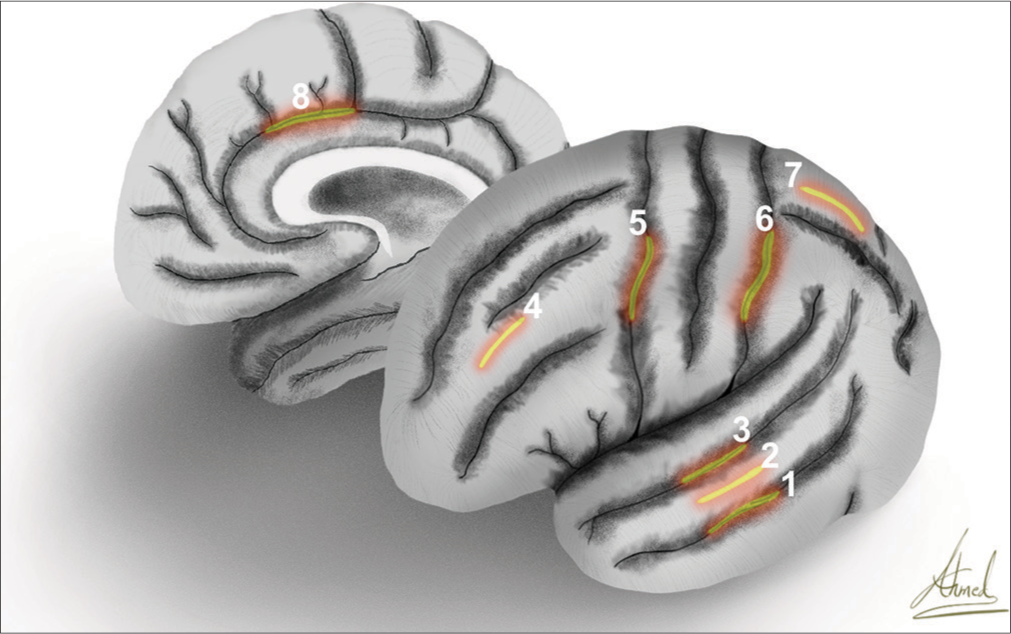
The most frequently accessed brain lobe was the temporal lobe (55.68%), and the most common location for cortical incision was through the middle temporal gyrus (71.09% of the transcortical-transtemporal cases). The second most common lobe accessed was the frontal lobe (24.66%) [
Figure 3:
Artistic depiction exhibits the lateral surface of the left hemisphere and medial surface of the right hemisphere with the marking of the cortical incisions of (1) inferior temporal sulcus, (2) middle temporal gyrus, (3) superior temporal sulcus, (4) middle frontal gyrus, (5) precentral sulcus, (6) postcentral sulcus, (7) superior parietal lobule, and (8) cingulate gyrus.
The anatomy of the incision was mainly parallel to gyri (62.1%) or unspecified (37.9%). The cortical incision length was primarily 3 cm (42.63%) and commonly located in the temporal lobe. The median length of the cortical incisions was 2 cm and ranged from 1 to 3 cm. Intraoperative image guiding systems, including diffusion tensor imaging and fiber tractography, were used in 46.01% of surgeries. The postoperative course was mainly reported as outcome measures, including good (55.52%), poor (28.38%), and death (14.62%). Among patients with poor outcomes, specific complications were recorded in 201 transcortical cases. Neurocognitive defects were the most common reported complication (27.23%), followed by hemiparesis (21.89%), seizures (19.8%), quadrantanopia (6.93%), and subdural hematoma (3.96%).
DISCUSSION
Transcortical approaches have been critical corridors for targeting intracranial lesions for many decades, but detailed information on these approaches remains scarce in the neurosurgical literature. In our review, we found that brain tumors, particularly gliomas, were the most frequent lesions accessed through these approaches, with the transcortical-transtemporal approach emerging as the most commonly reported route.
Navigating intra-axial lesions, the cortex, the underlying tract fibers, and the region’s eloquence deserve consideration. Evaluating the functional significance of both the cortical and subcortical zones, along with the objectives of the surgical approach, is critical for selecting the most suitable transcortical pathway. Regarding the location of the cortical access incision within the lobe, the temporal and frontal lobes seem to have advantages related to relatively less eloquent areas to be traversed. In this review, the primarily accessed lobe was the temporal lobe (55.68%), with the middle temporal gyrus being the most accessed area (71%). Ali et al.[
The frontal lobe was the second most frequent lobe (24.66%), mostly at the level of the middle frontal gyrus (53.41%). Lesions within these areas seem to be versatile, either intraparenchymal or ventricular. Other studies described more lobes to be accessed, including the parietal lobe (10.48%) and the cingulate gyrus (4.87%).
As regards the approach’ nomenclature, those comprised a variety of transcortical corridors classified according to the anatomical location and the target. The transtemporal are the most commonly reported approaches (70.48%) in our review, followed by transfrontal approaches (16.8%). The transtemporal pathway includes three main categories: proper transcorticaltranstemporal, transcortical amygdalohippocampectomy, and transtemporal trans ventricular. Those approaches are the highways that can be steered in trajectory according to the aimed location of the lesion.
Mazher et al.[
The pathologies treated with transcortical approaches have brain tumors representing the majority at 60.6% of the total intracranial pathologies. Those brain tumors are intraventricular more than intraparenchymal locations. The lateral ventricle represents the most involved ventricular cavity with approaching lesions within the frontal horn and the region around the foramen of Monro. Vascular lesions like AVMs comprised 8.18% of reported cases. Those AVMs that were operated using transcortical approaches include either parenchymal AVMs in the temporal lobe, followed by basal ganglia, thalamus, and insula, or periventricular/intraventricular AVMs mostly related to the atrium of the lateral ventricle.
Given the necessity for direct cortical incisions, transcortical approaches, notably for intra-axial lesions, may be linked to a heightened risk of complications. Conversely, the interhemispheric approach appears to be more suitable for pure cingulate gliomas, as evidenced by the successful complete tumor resection in nine out of ten patients, as reported by von Lehe and Schramm [
Multiple factors, including anatomical orientation, functional eloquence, and the characteristics of the lesion, primarily influence the selection of the specific surgical incision. The chosen technique may also vary depending on lesion-specific pathology, from gliomas to AVMs, ventricular lesions, and infections.[
Limitations
The selection of sulci, gyri, and subgyral incisions across the different studies may have been influenced by the surgeon’s expertise and the specific features of the lesion, thus needing to be addressed based on each case. The direction of the identifiable gyral incisions in the studies was mainly parallel to the gyri, and there is limited additional data to perform a comparison. In the included studies, there is a lack of data on this subject, as most studies have concentrated on the direction of the approach rather than the intricacies of the cortical access point description.
CONCLUSION
Transcortical approaches are essential techniques using specific cortical incisions as entry points. Intra-axial and intraventricular brain tumors, particularly gliomas, were the most frequently targeted pathologies. The selection of an incision length and location and surgical corridor depends on the lesion’s anatomical and functional features and specific pathology. It is critical to understand the intricacies of these approaches and individualize the technique based on each patient’s unique anatomy and peculiar lesion characteristics.
Ethical Approval
The Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent was not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation:
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
SUPPLEMENTARY FILE 1
References
1. Ali R, Englot DJ, Yu H, Naftel R, Haas KF, Konrad PE. Experience from 211 transcortical selective amygdalohippocampectomy procedures: Relevant surgical anatomy and review of the literature. Oper Neurosurg (Hagerstown). 2021. 21: 181-8
2. Anderson RC, Ghatan S, Feldstein NA. Surgical approaches to tumors of the lateral ventricle. Neurosurg Clin. 2003. 14: 509-25
3. Asgari S, Engelhorn T, Brondics A, Sandalcioglu IE, Stolke D. Transcortical or transcallosal approach to ventricle-associated lesions: A clinical study on the prognostic role of surgical approach. Neurosurg Rev. 2003. 26: 192-7
4. Barrow DL, Dawson R. Surgical management of arteriovenous malformations in the region of the ventricular trigone. Neurosurgery. 1994. 35: 1046-54
5. Broadway SJ, Ogg RJ, Scoggins MA, Sanford R, Patay Z, Boop FA. Surgical management of tumors producing the thalamopeduncular syndrome of childhood. J Neurosurg Pediatr. 2011. 7: 589-95
6. Brown DA, Hanalioglu S, Chaichana K, Duffau H. Transcorticosubcortical approach for left posterior mediobasal temporal region gliomas: A case series and anatomic review of relevant white matter tracts. World Neurosurg. 2020. 139: e737-47
7. Cao D, Chen Y, Guo Z, Ou Y, Chen J. Clinical outcome after microsurgical resection of central neurocytoma: A single-centre analysis of 15 years. Front Neurol. 2021. 12: 790641
8. Carmel PW. Tumours of the third ventricle. Acta Neurochir. 1985. 75: 136-46
9. Daglioglu E, Ergungor F, Polat E, Nacar O. Microsurgical resection of supratentorial cerebral cavernomas. Turk Neurosurg. 2010. 20: 348-52
10. Elkallaf MA, Elsaadany W, Moussa WM, Fayed AA. Transcortical approaches to large intraventricular tumors: A prospective case series of 20 patients. Egypt J Neurosurg. 2021. 36: 16
11. Ellenbogen RG. Transcortical surgery for lateral ventricular tumors. Neurosurg Focus. 2001. 10: E2
12. Faust K, Schmiedek P, Vajkoczy P. Approaches to temporal lobe lesions: A proposal for classification. Acta Neurochir (Wien). 2014. 156: 409-13
13. Gabarrós Canals A, Rodríguez-Hernández A, Young WL, Lawton MT. Temporal lobe arteriovenous malformations: Anatomical subtypes, surgical strategy, and outcomes. J Neurosurg. 2013. 119: 616-28
14. Gao Z, Qian L, Niu C, Chen B, Guo H, Sun P. Evacuating hypertensive intracerebral hematoma with a cortical sulcus approach. World Neurosurg. 2016. 95: 341-7
15. Gökalp HZ, Yüceer N, Arasil E, Deda H, Attar A, Erdoĝan A. Tumours of the lateral ventricle. A retrospective review of 112 cases operated upon 1970-1997. Neurosurg Rev. 1998. 21: 126-37
16. Hameed NF, Qiu T, Zhuang D, Lu J, Yu Z, Wu S. Transcortical insular glioma resection: Clinical outcome and predictors. J Neurosurg. 2018. 131: 706-16
17. He J, Li Z, Yu Y, Lu Z, Li Z, Gong J. Cognitive function assessment and comparison on lateral ventricular tumors resection by the frontal transcortical approach and anterior transcallosal approach respectively in children. Neurosurg Rev. 2020. 43: 619-32
18. Helmstaedter C, Van Roost D, Clusmann H, Urbach H, Elger CE, Schramm J. Collateral brain damage, a potential source of cognitive impairment after selective surgery for control of mesial temporal lobe epilepsy. J Neurol Neurosurg Psychiatry. 2004. 75: 323-6
19. Hoi SU. Microsurgical excision of paraventricular arteriovenous malformations. Neurosurgery. 1985. 16: 293-303
20. Howick J, Chalmers I, Glasziou P, Greenhalgh T, Heneghan C, Liberati A, editors. Explanation of the 2011 OCEBM levels of evidence-Centre for evidence-based medicine (CEBM). Oxford: University of Oxford; 2011. p.
21. Hussain K. Supratentorial ventricular tumors surgical management experience at Shaikh Zayed Hospital, Lahore. Headache. 2014. 9: 82
22. Ignacio MN, García-Rentería JA, Miguel SB, Vega FJ. Intraventricular cysticercosis. Neurosurgery. 1983. 12: 148-52
23. Juretschke FR, Güresir E, Marquardt G, Berkefeld J, Rosahl S, Klisch J. Trigonal and peritrigonal lesions of the lateral ventricle-surgical considerations and outcome analysis of 20 patients. Neurosurg Rev. 2010. 33: 457-64
24. Kaushik K, Choudhary A, Ahuja A, Varshney R, Sharma R. Camalote sign in intraventricular hydatid cyst: A rare presentation of uncommon disease. Surg Neurol Int. 2021. 12: 541
25. Kikuchi H, Miyamoto S, Nagata I, Taki W, Nakahara I. Surgical extirpation of the posterior hippocampal arteriovenous malformation. Surgical Neurol. 1997. 47: 251-6
26. Li Z, Li G, Liu Z, Pan Y, Hou Z, Wu L. Transcortical approach for insular gliomas: A series of 253 patients. J Neurooncol. 2020. 147: 59-66
27. Lutz MT, Clusmann H, Elger CE, Schramm J, Helmstaedter C. Neuropsychological outcome after selective amygdalohippocampectomy with transsylvian versus transcortical approach: A randomized prospective clinical trial of surgery for temporal lobe epilepsy. Epilepsia. 2004. 45: 809-16
28. Mazher S, Imran M, Ashraf J, Ahmed A, Shah IU, Zulfiqar F. Outcome of open transcortical approach in the management of intraventricular lesions. J Coll Physicians Surg Pak. 2013. 23: 857-61
29. Mittal S, Montes JL, Farmer JP, Rosenblatt B, Dubeau F, Andermann F. Long-term outcome after surgical treatment of temporal lobe epilepsy in children. J Neurosurg Pediatr. 2005. 103: 401-12
30. Miyagi Y, Shima F, Ishido K, Araki T, Kamikaseda K. Inferior temporal sulcus as a site of corticotomy: Magnetic resonance imaging analysis of individual sulcus patterns. Neurosurgery. 2001. 49: 1394-8
31. Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, editors. Chapter 7: Systematic reviews of etiology and risk. JBI Manual for evidence synthesis. South Australia: JBI; p. Available from: ??? [Last accessed on 2021 Oct 20]
32. Morshed RA, Young JS, Han SJ, Hervey-Jumper SL, Berger MS. The transcortical equatorial approach for gliomas of the mesial temporal lobe: Techniques and functional outcomes. J Neurosurg. 2019. 130: 822-30
33. Nair S, Rout D, Menon G, Kachhara R, Bhattacharya RN. Medial trigonal arteriovenous malformations. Keio J Med. 2000. 49: 14-9
34. Olivier A. Transcortical selective amygdalohippocampectomy in temporal lobe epilepsy. Can J Neurol Sci. 2000. 27: S68-76
35. Ozek MM, Ture U. Surgical approach to thalamic tumors. Child’s Nerv Syst. 2002. 18: 450-6
36. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int J Surg. 2021. 88: 105906
37. Park ES, Cho YH, Kim JH, Kim SJ, Khang SK, Kim CJ. Frontal transcortical approach in 12 central neurocytomas. Acta Neurochir. 2012. 154: 1961-71
38. Pendl G, Öztürk E, Haselsberger K. Surgery of tumours of the lateral ventricle. Acta Neurochir. 1992. 116: 128-36
39. Potts MB, Young WL, Lawton MT. Deep arteriovenous malformations in the basal ganglia, thalamus, and insula: Microsurgical management, techniques, and results. Neurosurgery. 2013. 73: 417-29
40. Roszkowski M, Drabik K, Barszcz S, Jozwiak S. Surgical treatment of intraventricular tumors associated with tuberous sclerosis. Childs Nerv Syst. 1995. 11: 335-9
41. Schaller C, Jung A, Clusmann H, Schramm J, Meyer B. Rate of vasospasm following the transsylvian versus transcortical approach for selective amygdalohippocampectomy. Neurol Res. 2004. 26: 666-70
42. Schijman E, Monges J, Raimondi AJ, Tomita T. Choroid plexus papillomas of the III ventricle in childhood: Their diagnosis and surgical management. Childs Nerv Syst. 1990. 6: 331-4
43. Schramm J, Aliashkevich AF. Surgery for temporal mediobasal tumors: Experience based on a series of 235 patients. Neurosurgery. 2008. 62: 1272-82
44. Seçer HI, Bülent DÜ, Yusuf İZ, Tehli O, Solmaz I, Gönül E. Tumors of the lateral ventricle: The factors that affected the preference of the surgical approach in 46 patiens. Turk Neurosurg. 2008. 18: 345-55
45. Sharifi G, Hallajnejad M, Dastgheib SS, Lotfinia M, Mirghaed OR, Amin AM. Clinical outcome of selective amygdalectomy in a series of patients with resistant temporal lobe epilepsy. Surg Neurol Int. 2021. 12: 575
46. Shi ZF, Sun DL, Song JP, Yu Y, Ying M. Emotion and cognitive function assessment of patients with central neurocytoma resection through transcortical frontal approach: A 5-year postoperative follow-up study. Chin Med J (Engl). 2011. 124: 2593-8
47. Shimizu H, Maehara T. Modification of peri-insular hemispherotomy and surgical results. Neurosurgery. 2000. 47: 367-73
48. Thudium MO, Campos AR, Urbach H, Clusmann H. The basal temporal approach for mesial temporal surgery: Sparing the Meyer loop with navigated diffusion tensor tractography. Oper Neurosurg. 2010. 67: ons385-90
49. Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann Int Med. 2018. 169: 467-73
50. Villemure JG, Mascott CR. Peri-insular hemispherotomy: Surgical principles and anatomy. Neurosurgery. 1995. 37: 975-81
51. Vivas AC, Reintjes S, Shimony N, Vale FL. Surgery of the amygdala and uncus: A case series of glioneuronal tumors. Acta Neurochir (Wien). 2020. 162: 795-801
52. Von Lehe M, Schramm J. Gliomas of the cingulate gyrus: Surgical management and functional outcome. Neurosurg Focus. 2009. 27: E9
53. Waga S. Surgical treatment of arteriovenous malformations in the lateral ventricle. Neurol Res. 1986. 8: 18-24
54. Wang X, Liang H, Xu M, Shen G, Xu L. Comparison between transsylvian-transinsular and transcortical-transtemporal approach for evacuation of intracerebral hematoma. Acta Cir Bras. 2013. 28: 112-8
55. Zhang HT, Xue S, Li PJ, Fu YB, Xu RX. Treatment of huge hypertensive putaminal hemorrhage by surgery and cerebrospinal fluid drainage. Clin Neurol Neurosurg. 2013. 115: 1602-8
56. Zhou H, Miller D, Schulte DM, Benes L, Rosenow F, Bertalanffy H. Transsulcal approach supported by navigation-guided neurophysiological monitoring for resection of paracentral cavernomas. Clin Neurol Neurosurg. 2009. 111: 69-78
57. Zhu W, He J, Li X, Wang L, Lu Z, Li C. Cognitive performance change of pediatric patients after conducting frontal transcortical approach to treat lateral ventricular tumor. Childs Nerv Syst. 2017. 33: 2099-108