- Department of Neurosurgery, Mahatma Gandhi University of Medical Sciences and Technology, Jaipur, Rajasthan, India.
- Department of Neuroanesthesia, Mahatma Gandhi University of Medical Sciences and Technology, Jaipur, Rajasthan, India.
Correspondence Address:
Anmol Singh Randhawa, Department of Neurosurgery, Mahatma Gandhi University of Medical Sciences and Technology, Ricco Industrial Area, Jaipur, Rajasthan, India.
DOI:10.25259/SNI_278_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Anurag Srivastava1, Anmol Anant Dobriyal1, Anmol Singh Randhawa1, Pavan Kumar Jain1, Shiteez Agrawal1, Jitendra Singh Verma1, Pankaj Gupta1, Bhawani Shanker Sharma1, Yogesh Agrawal1, Medha Bhardwaj2. Retrospective analysis of the outcomes of endoscopic transsphenoidal surgery for Cushing’s disease. 12-Jul-2024;15:240
How to cite this URL: Anurag Srivastava1, Anmol Anant Dobriyal1, Anmol Singh Randhawa1, Pavan Kumar Jain1, Shiteez Agrawal1, Jitendra Singh Verma1, Pankaj Gupta1, Bhawani Shanker Sharma1, Yogesh Agrawal1, Medha Bhardwaj2. Retrospective analysis of the outcomes of endoscopic transsphenoidal surgery for Cushing’s disease. 12-Jul-2024;15:240. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=12989
Abstract
Background: The first-line surgical management of an adrenocorticotropic hormone (ACTH)-secreting pituitary adenoma causing Cushing’s disease (CD) is endoscopic transsphenoidal resection of the tumor. This study was performed to assess postoperative (postop) complications and remission in endoscopic surgically resected cases of CD.
Methods: Data of patients who underwent endoscopic transsphenoidal surgery (ETSS) for CD were collected from the neurosurgery department at a tertiary care center in a retrospective manner from January 2015 to February 2022 and analyzed. Postoperative remission was categorized as – early morning serum cortisol
Results: A total of 41 patients were identified who underwent 44 ETSS during the same timeframe. Preoperative magnetic resonance imaging localized an adenoma in all 41 patients, out of which 32 were microadenoma, and nine were macroadenoma (2 with cavernous sinus invasion). Intrapetrosal sinus sampling was performed in 35 (85%) patients. The rate of remission for the initial surgery was 85.4% using the standard criteria and 68.3% using strict criteria. Three patients underwent early repeat surgery for the persistent disease as the day 3 cortisol was high (306–555 nmol/L). Once the outcome of this surgery was also included, the overall rate of remission was 90.2% (37/41). None of the patients had meningitis, cerebrospinal fluid leakage, visual deterioration, or vascular injury. Permanent and transient diabetes insipidus (DI) occurred in 9.75% and 26.8% following the first ETSS, respectively. We also noted a single case of CD recurrence in 9 months during the total follow-up period of 84 months.
Conclusion: ETSS has satisfactory rates of remission for the primary treatment of CD, with rates being higher for microadenomas. A long follow-up period is needed to assess the rates of recurrence. Patients must be counseled regarding the risk of postop DI, whether transient or permanent, as a possible complication.
Keywords: Cushing’s disease (CD), Diabetes insipidus, Endoscopic transsphenoidal surgery (ETSS), Microadenoma, Pituitary macroadenoma
INTRODUCTION
Cushing’s disease (CD) is a rare condition with limited epidemiologic data available. Its estimated prevalence is around 40/million, and incidence ranges from 1.2 to 2.4/million/year, as per various studies.[
The recurrence and remission rates post-ETSS for CD vary vastly as per the criteria for defining remission,[
MATERIALS AND METHODS
Study design
Retrospective analysis of prospectively collected data of CD patients operated in the department of neurosurgery at a tertiary care center, through image-guided endoscopic transsphenoidal approach was conducted. Biochemical and clinical data were gathered over 7 years (84 months–January 2015 to February 2022), and the follow-up period was reviewed.
Study population
Screening for CD was done after the identification of characteristic clinical features [
Inadequate cortisol suppression – <50 nmol/L – after an overnight dexamethasone suppression test (ONDST); OR Raised late-night salivary cortisol (LNSF) level; OR Raised 24 h urinary free cortisol (UFC) level.
According to standard guidelines,[
After an initial suspicion of Cushing syndrome, the following evaluation protocol was followed[
Rule out/exclude exogenous glucocorticoid exposure Initial screening tests (anyone to be performed) 24 h UFC -×3 the normal value (≥2 tests) LNSF - >5.5 nmol/L (≥2 tests) Loss of circadian rhythm of cortisol secretion 1 mg ONDST On day 1 at 11 pm, 1mg dexamethasone was administered, and on day 2 at 8 am, cortisol value assessed – >49.6 nmol/L (Single test) Longer low-dose dexamethasone suppression test (DST) (2 mg/d for 48 h). This is done in obese/PCOS/metabolic syndrome/pseudo-Cushing’s 0.5 mg four tablets of dexamethasone are given 6 h apart on days 1 and 2. On day 3 at 8 am, if S. cortisol >1.8 mcg/dL – endogenous CD (single test). If any abnormal test – Evaluation for the endogenous cause of hypercortisolism
9 am plasma ACTH – sample maintained in the cold chain, transported in the cold chain, and centrifuged.
ACTH >20 pg/mL – Confirmatory for ACTH dependent ACTH <10 pg/mL – ACTH independent ACTH >90 pg/mL – Ectopic ACTH syndrome
Surgical procedure
All of the ETSS procedures were carried out by three senior neurosurgeons super- specializing in endoscopic anterior skull base and pituitary surgeries. It consists of a binostril endoscopic transsphenoidal approach. Selective adenomectomy was done on all the patients with adenomas identified on the preoperative MRI scans. Confirmation of diagnosis of ACTH-secreting adenoma or hyperplasia was done by immunohistochemical staining for pituitary hormones and histopathological examination of the postop specimens.
Postop assessment
Empirical oral hydrocortisone was given to the patients on postop day 1 and on the morning of postop day 2 before the assessment of early morning serum cortisol on postop day 3. Sampling for the same was done at 8 am on the 3rd day if the patient was clinically stable before the hydrocortisone was administered.
The Endocrine Society Clinical Practice Guideline defines postoperative biochemical remission as morning serum cortisol <138 nmol/L (5 μg/dL) within 7 postop days postoperatively[
In case the serum cortisol on postop day 3 is between 50 and 138 nmol/L, daily serial sampling is done to identify whether the cortisol is falling further or not, and an assessment of improvement or resolution of the clinical sequelae of hypercortisolemia is made (like improvement in glycemic control or blood pressure) before a repeat ETSS is taken into consideration.[
Transient cranial diabetes insipidus (DI) was defined as the development of hypotonic polyuria in the postop period that required at least a single dose of desmopressin,[
Statistics
Data are expressed as range (median) and percentage (number of patients). Fishers’ Exact test was utilized for comparison of the categorical variables among the two groups. The P-value was considered statistically significant at less than 0.05. Statistical analysis was done using the Statistical Package for the Social Sciences Software.
RESULTS
Demographics
Forty-four endoscopic transsphenoidal procedures were performed in 41 patients. Median (range) age was 33.6 years (14–71), out of which 29 were female and 12 were male. Median (range) duration of symptoms was 39 months (6– 84), among which 73% (30/41) had type 2 diabetes mellitus and 95% (39/41) had hypertension.
Preoperative imaging and IPSS
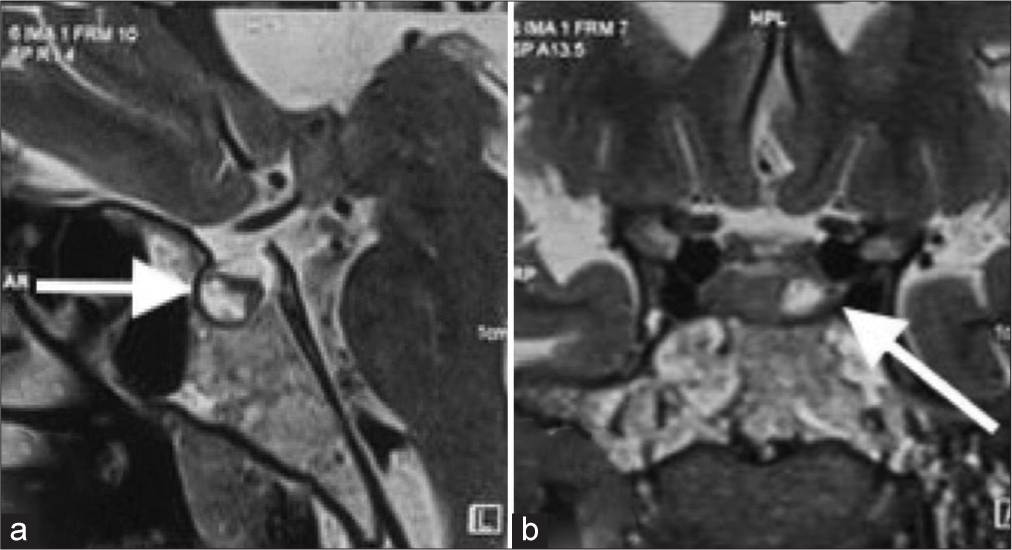
Preoperative MRI [
Figure 2:
(a) Sagittal magnetic resonance imaging (MRI) brain showing a hypoenhancing lesion (white arrow) in the left half of the pituitary gland, measuring approximately 9 × 8 × 7.2 mm. (b) Coronal MRI brain showing heterogeneously hyperintense lesion in the pituitary gland, on T2 with multiple peripheral T2 hypointense foci, suggestive of microadenoma (white arrow).
Postop remission
Postoperative outcomes are summarized as follows:
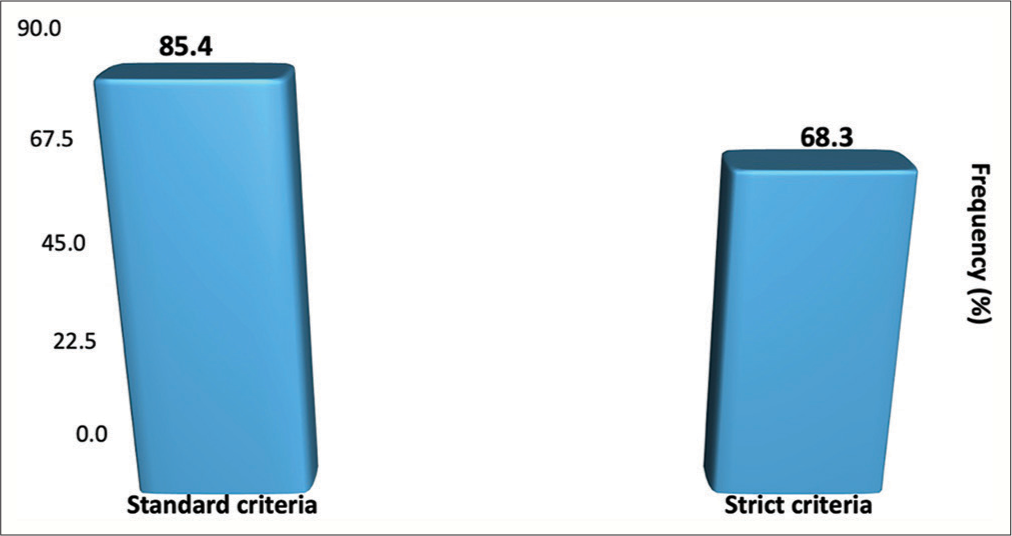
Using the standard criteria (8 am postop day 3 serum cortisol <138 nmol/L within 7 postop days and an improvement in the clinical features of hypercortisolism), the rate of remission in the postop period for initial surgery was 85.4% (35/41) for the whole group and 81.2% (26/32) if patients with macroadenomas were excluded from the study. Three patients had to undergo early repeat ETSS for persistent CD; postop day 3, serum cortisol levels ranged from 306 to 555 nmol/L. Including the outcome of repeat early ETSS, the overall remission rate of remission was 90.2% (37/41) [
Using the strict criteria of early remission (postop day 3 serum cortisol levels <50 nmol/L), the overall postop rate of remission was 68.3% (28/41) [
On day 3, 11 patients (26.8%) had serum cortisol between 50 and 138 nmol/L, out of which 7 received metyrapone therapy before the surgery. Six patients had daily serial measurements of 8 am serum cortisol till postop 14 days, and it was observed that it declined after day 3 in all six patients.
Persistent disease
Six patients (14.6%) had persistent hypercortisolemia after initial ETSS. Three patients had to undergo an early repeat endoscopic TSS. The rate of remission after this repeat early ETSS was 66% (2/3) using the standard criteria and 33% (1/3) using strict criteria. Out of the patients having persistent disease after the repeat surgery, radiosurgery was performed on 1 patient, while the other patient was started on medical therapy.
Postop complications
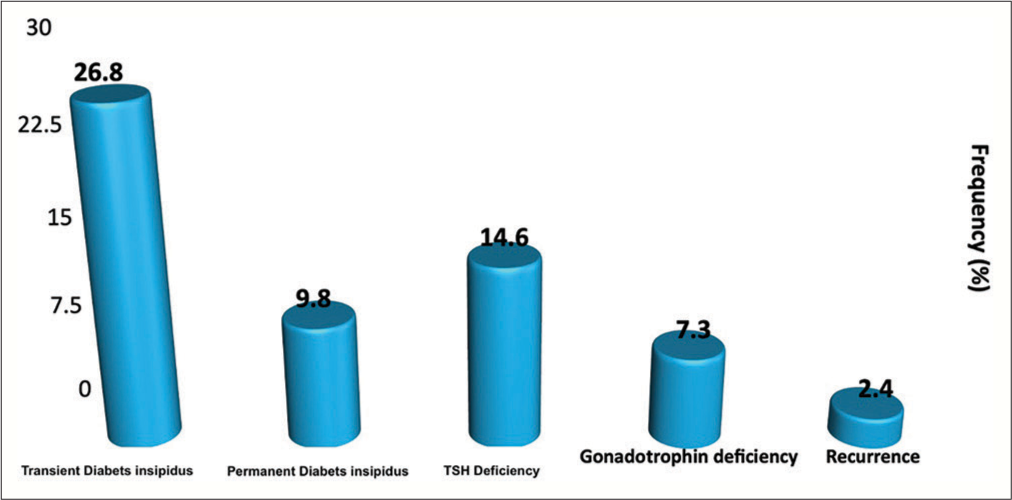
After the initial ETSS, among the 41 patients, 11 patients developed transient DI (26.8%), whereas four patients developed permanent DI (9.75%). Postoperatively, we observed 6 cases (14.6%) of new-onset TSH deficiency and 3 cases (7.3%) of gonadotropin deficiency (in premenopausal females) [
Recurrence
None of our patients were lost to follow-up. The median range of duration of follow-up was 4 months, over which one patient had a recurrence of CD. Preoperative MRI showed a macroadenoma; serum cortisol on day 3 after the initial ETSS was 71 nmol/L, which fulfilled the standard criteria for remission but not the more strict criteria. The patient underwent a second ETSS 9 months later. No tumor was visible intraoperatively, so no tissue was removed. Day 3 serum cortisol concentration was 308 nmol/L, and the patient was commenced on a trial of metyrapone.
An overall schema of management of the CD cases in our series is depicted in
DISCUSSION
There is a varied difference among the rates of remission after an ETSS among CD patients, mainly due to the variations in the criteria used to define remission.[
In this study, we report two different remission rates using these two criteria, which are widely accepted. Our rate of remission rate, including the patients who underwent an early second ETSS, according to the standard guidelines, is 90.2%, which is at par with the other large-scale studies.[
Evidence also suggests that the higher postop day 3 cortisol level is associated with a higher risk of CD recurrence. A retrospective cohort study of 81 CD patients undergoing ETSS conducted by Mayberg et al.,[
While our data are encouraging when compared to other studies on the recurrence of CD that have published rates up to 22%,[
The most common clinical finding seen in CD is centripetal obesity, which is nonspecific and has a poor discriminatory value.
The Endocrine Society[ Central obesity with any feature of protein catabolism Osteoporosis/hypertension at a young age Children with decreasing height percentile and increasing weight Any incidentally detected adrenal mass – can be functional – the most likely cause is Cushing’s Presence of obesity, hypertension, diabetes mellitus, acne, and hirsutism.
We have mentioned the clinical features of our study, as shown in
There was a very low postop surgical complication rate in our study, and we did not have any cases of vascular injury, CSF leak, or visual compromise. Many other case series have reported an incidence of 0–7.2% of CSF leakage and 0–7.9% for meningitis.[
While our surgical complication rate was very low, the rate of endocrine complications was similar to the one reported in many other studies, especially in the case of DI. Permanent DI was seen in 9.8% of cases, and transient DI in 26.8% of cases. The comparatively high rate of transient DI might be due to diagnostic criteria used in our practice, as we defined transient postop DI as a single episode of hypotonic polyuria in the presence of elevated or normal plasma sodium levels, which required at least 1 dose of desmopressin. On the contrary, some studies report that any polyuria that lasts for <2 days is transient DI [
Strengths and limitations
We have utilized two widely accepted criteria to report the two remission rates, which is the strength of our series. All the ETSS were performed by three qualified senior pituitary surgeons, which removes the bias of surgeon experience. The disadvantage of our study is the low sample size. Furthermore, we included patients who were only recently operated on, to maximize the numbers for analysis of post-surgical complications. Along with that, we did not have full data on the longitudinal postoperative results since it was a retrospective study, and it highlights the requirement for a standardized follow-up for consistency in reporting the results.
CONCLUSION
ETSS in patients with CD offers an excellent remission rate and very low rates of morbidity. The rates of remission are much higher when the standard criteria of early morning serum cortisol of <138 nmol/L, within postop 7 days, are compared with postop day 3 cortisol and assessed whether it is <50 nmol/L or not. A higher rate of remission was found in patients with microadenoma. It is extremely necessary to counsel patients regarding the risk of postop endocrine deficiencies, with special emphasis on permanent DI. A longer follow-up period is needed to assess the rates of recurrence accurately.
Ethical approval
Ethical approval was not required as per our instituitional guidelines.
Declaration of patient consent
Patient’s consent was not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
Acknowledgment
We want to thank the Departments of Neurosurgery, Radiology, and Endocrinology for providing us with the necessary data for this study to be conducted and our Head of Department for guiding and motivating us to conduct this study.
References
1. Adams JR, Blevins LS, Allen GS, Verity DK, Devin JK. Disorders of water metabolism following transsphenoidal pituitary surgery: A single institution’s experience. Pituitary. 2006. 9: 93-9
2. Ammirati M, Wei L, Ciric I. Short-term outcome of endoscopic versus microscopic pituitary adenoma surgery: A systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2013. 84: 843-9
3. Atkinson AB, Kennedy A, Wiggam MI, McCance DR, Sheridan B. Long-term remission rates after pituitary surgery for Cushing’s disease: The need for long-term surveillance. Clin Endocrinol. 2005. 63: 549-59
4. Barzaghi LR, Medone M, Losa M, Bianchi S, Giovanelli M, Mortini P. Prognostic factors of visual field improvement after trans-sphenoidal approach for pituitary macroadenomas: Review of the literature and analysis by quantitative method. Neurosurg Rev. 2012. 35: 369-78
5. Berker M, Işikay I, Berker D, Bayraktar M, Gürlek A. Early promising results for the endoscopic surgical treatment of Cushing’s disease. Neurosurg Rev. 2014. 37: 105-14
6. Biller BM, Grossman AB, Stewart PM, Melmed S, Bertagna X, Bertherat J. Treatment of adrenocorticotropin-dependent Cushing’s syndrome: A consensus statement. J Clin Endocrinol Metab. 2008. 93: 2454-6
7. Bolland MJ, Holdaway IM, Berkeley JE, Lim S, Dransfield WJ, Conaglen JV. Mortality and morbidity in Cushing’s syndrome in New Zealand. Clin Endocrinol. 2011. 75: 436-42
8. Brady Z, Garrahy A, Carthy C, O’Reilly MW, Thompson CJ, Sherlock M. Outcomes of endoscopic transsphenoidal surgery for Cushing’s disease. BMC Endocr Disord. 2021. 21: 36
9. Broersen LH, Biermasz NR, van Furth WR, de Vries F, Verstegen MJ, Dekkers OM. Endoscopic vs. microscopic transsphenoidal surgery for Cushing’s disease: A systematic review and meta-analysis. Pituitary. 2018. 21: 524-34
10. Cebula H, Baussart B, Villa C, Assié G, Boulin A, Foubert L. Efficacy of endoscopic endonasal transsphenoidal surgery for Cushing’s disease in 230 patients with positive and negative MRI. Acta Neurochir (Wien). 2017. 159: 1227-36
11. Dehdashti AR, Gentili F. Current state of the art in the diagnosis and surgical treatment of Cushing disease: Early experience with a purely endoscopic endonasal technique. Neurosurg Focus. 2007. 23: E9
12. Fahlbusch R, Buchfelder M, Müller OA. Transsphenoidal surgery for Cushing’s disease. J R Soc Med. 1986. 79: 262-9
13. Garrahy A, Moran C, Thompson CJ. Diagnosis and management of central diabetes insipidus in adults. Clin Endocrinol. 2019. 90: 23-30
14. Glynn N, Agha A. Diagnosing growth hormone deficiency in adults. Int J Endocrinol. 2012. 2012: 972617
15. Jho HD, Carrau RL. Endoscopic endonasal transsphenoidal surgery: Experience with 50 patients. J Neurosurg. 1997. 87: 44-51
16. Lila AR, Sarathi V, Jagtap VS, Bandgar T, Menon P, Shah NS. Cushing’s syndrome: Stepwise approach to diagnosis. Indian J Endocr Metab. 2011. 15: 317-21
17. Magro E, Graillon T, Lassave J, Castinetti F, Boissonneau S, Tabouret E. Complications related to the endoscopic Endonasal Transsphenoidal approach for nonfunctioning pituitary macroadenomas in 300 consecutive patients. World Neurosurg. 2016. 89: 442-53
18. Mamelak AN, Carmichael J, Bonert VH, Cooper O, Melmed S. Single-surgeon fully endoscopic endonasal transsphenoidal surgery: Outcomes in three-hundred consecutive cases. Pituitary. 2013. 16: 393-401
19. Mayberg M, Reintjes S, Patel A, Moloney K, Mercado J, Carlson A. Dynamics of postoperative serum cortisol after transsphenoidal surgery for Cushing’s disease: Implications for immediate reoperation and remission. J Neurosurg. 2018. 129: 1268-77
20. McCance DR, Besser M, Atkinson AB. Assessment of cure after transsphenoidal surgery for Cushing’s disease. Clin Endocrinol (Oxf). 1996. 44: 1-6
21. Mortini P, Losa M, Barzaghi R, Boari N, Giovanelli M. Results of transsphenoidal surgery in a large series of patients with pituitary adenoma. Neurosurgery. 2005. 56: 1222-33
22. Nemergut EC, Zuo Z, Jane JA, Laws ER. Predictors of diabetes insipidus after transsphenoidal surgery: A review of 881 patients. J Neurosurg. 2005. 103: 448-54
23. Nieman LK, Biller BM, Findling JW, Hassan Murad M, Newell-Price J, Savage MO. Treatment of Cushing’s syndrome: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015. 100: 2807-31
24. Nishioka H, Yamada S. Cushing’s disease. J Clin Med. 2019. 8: 1951
25. Nomikos P, Buchfelder M, Fahlbusch R. Current management of prolactinomas. J Neurooncol. 2001. 54: 139-50
26. Patil CG, Prevedello DM, Lad SP, Vance ML, Thorner MO, Katznelson L. Late recurrences of Cushing’s disease after initial successful transsphenoidal surgery. J Clin Endocrinol Metab. 2008. 93: 358-62
27. Petersenn S, Beckers A, Ferone D, van der Lely A, Bollerslev J, Boscaro M. Therapy of endocrine disease: Outcomes in patients with Cushing’s disease undergoing transsphenoidal surgery: Systematic review assessing criteria used to define remission and recurrence. Eur J Endocrinol. 2015. 172: R227-39
28. Sarkar S, Rajaratnam S, Chacko G, Mani S, Hesargatta AS, Chacko AG. Pure endoscopic transsphenoidal surgery for functional pituitary adenomas: Outcomes with Cushing’s disease. Acta Neurochir. 2016. 158: 77-86
29. Seckl J, Dunger D. Postoperative diabetes insipidus. Br Med J. 1989. 298: 2
30. Sharma ST, Nieman LK, Feelders RA. Comorbidities in Cushing’s disease. Pituitary. 2015. 18: 188-94
31. Starke RM, Reames DL, Chen CJ, Laws ER, Jane JA. Pure endoscopic transsphenoidal surgery for Cushing’s disease: Techniques, outcomes, and predictors of remission. Neurosurgery. 2013. 72: 240-7 discussion 247
32. Storr H, Alexandraki K, Martin L, Isidori AM, Kaltsas GA, Monson JP. Comparisons in the epidemiology, diagnostic features and cure rate by transsphenoidal surgery between paediatric and adult-onset Cushing’s disease. Eur J Endocrinol. 2011. 164: 667-74
33. Trainer PJ, Lawrie HS, Verhelst J, Howlett TA, Lowe DG, Grossman AB. Transsphenoidal resection in Cushing’s disease: Undetectable serum cortisol as the definition of successful treatment. Clin Endocrinol. 1993. 38: 73-8
34. Wagenmakers MA, Boogaarts HD, Roerink SH, Timmers HJ, Stikkelbroeck NM, Smit JW. Endoscopic transsphenoidal pituitary surgery: A good and safe primary treatment option for Cushing’s disease, even in case of macroadenomas or invasive adenomas. Eur J Endocrinol. 2013. 169: 329-37
35. Yap LB, Turner HE, Adams CB, Wass JA. Undetectable postoperative cortisol does not always predict long-term remission in Cushing’s disease: A single Centre audit. Clin Endocrinol. 2002. 56: 25-31