- Professor of Clinical Neurosurgery, School of Medicine, State University of NY at Stony Brook and Editor-in-Chief Surgical Neurology International NY, USA, and c/o Dr. Marc Agulnick, 1122 Franklin Avenue Suite 106, Garden City, NY, USA
- Assistant Clinical Professor of Orthopedics, NYU Langone Hospital, Long Island, NY, USA, 1122 Franklin Avenue Suite 106, Garden City, NY, USA
Correspondence Address:
Nancy E. Epstein, M.D., F.A.C.S., Professor of Clinical Neurosurgery, School of Medicine, State University of NY at Stony Brook, and Editor-in-Chief of Surgical Neurology International NY, USA, and c/o Dr. Marc Agulnick, 1122 Franklin Avenue Suite 106, Garden City, NY, USA
DOI:10.25259/SNI_701_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Nancy E. Epstein1, Marc A. Agulnick2. Perspective: Risks/adverse events for epidural spinal injections. 13-Sep-2024;15:328
How to cite this URL: Nancy E. Epstein1, Marc A. Agulnick2. Perspective: Risks/adverse events for epidural spinal injections. 13-Sep-2024;15:328. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13096
Abstract
Background: Despite the lack of FDA (Food and Drug Administration) approval, cervical and lumbar epidural spinal injections are frequently performed in the US to address back pain and/or painful radiculopathy. The three major types of injections include; interlaminar/translaminar (ESI), transforaminal (TFESI), or caudal injections. Notably, most studies document little to no clear short-term, and no long-term benefits/efficacy for these injections vs. various placebos.
Methods: More adverse events (AE) occurred with cervical© rather than lumbar (L) injections, and more severe AE were attributed to C-TFESI vs. CESI injections.
Results: Acute post injection AE symptoms were observed immediately or within 72 post-injection hours. These symptoms included; hypotension, acute respiratory distress, chest pain, upper extremity numbness, weakness, paresthesias, paralysis, and fevers. More AE were attributed to cervical C-TFESI vs. cervical CESI. These AE included; intramedullary/cord injections, intravascular injections (i.e. vertebral artery) resulting in brain stem/cerebellar/cord strokes, epidural abscess/infection, confusion, epidural hematomas, intracranial hypotension, and/or 6th nerve cranial palsies. AE for lumbar LESI/L-TFESI included; infections/abscess, epidural hematomas/subdural hematomas, intravascular injections, cerebrospinal fluid (CSF) leaks/dural tears (DT), and intracranial/postural hypotension. Notably, the vast majority of studies showed little to no short-term, and no long-term benefits for cervical or lumbar ESI/TFESI vs placebos (i.e. mostly consisting of normal saline alone, or saline plus local anesthesia).
Conclusion: Epidural cervical and lumbar ESI or TFESI spinal injections demonstrated minimal to no short-term, and no long-term benefits for the treatment of cervical and/or lumbar pain/radiculopathy vs. placebos. Further, more AE were observed for cervical vs. lumbar epidural injections overall, with more AE usually seen with TFESI vs. ESI procedures.
Keywords: Spinal Epidural Injections: Interlaminar, Translaminar (ESI), Transforaminal (TFESI), Caudal: Cervical, Lumbar, Adverse Events, Cord Injections, Cerebrospinal Fluid (CSF) Leaks, Neurological Deficits, Paralysis, Vascular Injections
INTRODUCTION
Despite the lack of FDA (Food and Drug Administration) approval, Medicare reported that over 9 million cervical and lumbar epidural spinal injections were performed in 2012 [
AE Attributed to ESI/TFESI That Are Not FDA (Food and Drug Administration) Approved
Two articles discussed the lack of FDA approval of cervical or lumbar spinal epidural injections.[
Time of Onset of Acute/Symptoms/Signs After Cervical/Lumbar ESI/TFESI Injections
Schreiber et al. in 2016 reported that adverse symptoms/signs occurred immediately to within 72 hours following epidural injections; these typically included hypotension, acute respiratory distress, chest pain, upper extremity numbness, weakness, paresthesias, and/or paralysis, and fever [
Minimal or No Short-Term and No Long-Term Benefits of Cervical ESI/TFESI
Two studies documented minimal/no short-term, and no long-term benefits of cervical ESI/TFESI vs. placebos [
Minimal to No Short-Term and No Long-Term Benefits of Lumbar ESI, TFESI, or Caudal Injections vs. Placebos
Five studies documented minimal/no short-term, and no long-term benefits of lumbar ESI/TFESI [
More Minor vs. Major AE Reported for Cervical and/or Lumbar ESI, TFESI, or Caudal Lumbar Injections
Five studies documented more minor vs. major AE attributed to cervical and/or lumbar epidural injections; additionally some reports stated more AE occurred following TFESI vs. ESI [
Variable Reporting of AE for Cervical and/or Lumbar ESI vs TFESI, with 2 of 3 Studies Emphasizing More AE with Cervical Injections
Three studies reported different frequencies for AE attributed to cervical and/or lumbar ESI vs. TFESI, with 2 citing greater AE for cervical injections (ESI/TFESI) [
Various Symptoms/Signs of DT/CSF Leaks and Other AE Following Preoperative Spinal Epidural Injections
Multiple symptoms and signs may signal DT/CSF leaks or other AE following preoperative cervical and/or lumbar epidural injections [
Frequency and Treatment/Repair of Traumatic Dural Tears (DT) Attributed to Preoperative Cervical/Lumbar Epidural Steroid Injections
Epstein documented in multiple studies that preoperative cervical and/or lumbar epidural injections resulted in intraoperatively documented DT that warranted direct repair [
High Failure Rate for Epidural Blood Patches (EBP) Utilized to Occlude Lumbar DT/CSF Leaks Largely Attributed to Preoperative ESi/TFESI and Other Factors
In 2023, Epstein emphasized the need for early direct repair of MR/Myelo-CT-documented sites of DT/CSF leaks encountered intraoperatively following preoperative ESI and other procedures/factors (i.e. lumbar punctures, spinal anesthesia (SA), and spontaneous intracranial hypotension (SICH)) rather than choosing to perform EBP [
Incidence of Acute Cervical Spinal Cord Injuries (SCI) Due to ESI/TFESI
Schreiber (2016) documented that 7 (0.52%) of 1343 patients admitted to an acute spinal cord injury (SCI) center sustained cervical injures attributed to ESI/TFESI (2001-2008) [
Frequency of Epidural Hematomas Due to Epidural Injections/ESI
Out of a total of 1182 (0.51%) cervical and 4617 (0.15%) lumbar interlaminar ESI performed over an 8-year period, Smith et al. (2017) observed that 13 patients required emergency neurosurgery for 3 DT/CSF leaks, 3 infections, and 7 hematomas [
Case Report: 3 Month Delay in L23 Laminectomy Due to Administration of 3 Lumbar ESI
In a 2015 case study, Epstein (2015) presented a 54-year-old-male with an MR-documented massive L23 disc herniation filling the spinal canal who was negligently treated for 3 months with ESI (i.e., 1 per month); when he finally presented paraplegic, he underwent a L23 laminectomy and fortunately recovered significant neurological function [
CONCLUSION
In this perspective/review of the literature, patients undergoing epidural cervical and lumbar ESI or TFESI spinal injections demonstrated minimal to no short-term, and no long-term benefits for the treatment of cervical and/or lumbar pain/radiculopathy vs. placebos. Further, more AE were observed for cervical vs. lumbar epidural injections, with more frequent and severe AE seen with TFESI vs. ESI procedures.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Commentary
Author: Jamie L. Baisden MD (Neurosurgery)
This article is a bit heavy on the risks of ESI and TFESI. What is this paper’s overall purpose? To let people know the dangers of epidural steroid injections lacking FDA approval, or to save insurance companies money? As spine surgeons, we should not allow insurance companies that don’t have a medical degree to act as gait-keepers to determine who should undergo multiple non-FDA-approved spinal epidural injections before being “disqualified” or “qualified” for spine surgery. Most oral steroids (i.e., oral steroids - Medrol Dose Packs, or Prednisone) or intramuscular steroid injections (i.e., especially trigger point injections) have minimal risks/minimal down-sides and may make people feel better, particularly in the short-term (i.e., weeks). Notably, the placebo effect of any injection (i.e., many studies typically compare epidural steroid vs Normal Saline epidural or intramuscular injections) is often around 30%; so, you may want to concede that 30% of patients may feel some transient improvement in the first 1-2 weeks. More critically, however, the “natural history” of spontaneous improvement kicks in at around 3-4 weeks, just around the time the “benefits” of steroids are actually waning or disappearing; patients may then mistakenly attribute their continued “improvement” to the steroids, rather than to the natural course of symptom resolution.
My primary concern, however, is what is left for patients if we can’t offer narcotics or epidural steroid injections anymore, and the patient can’t take NSAIDs (i.e., on blood thinners for cardiovascular disease, and/or a history of gastrointestinal, and/or chronic kidney disease)? We can certainly offer patient education and a multitude of medications (i.e., muscle relaxants, neuromodulators, non-opioid/non-NSAID medications) and/or other non-invasive modalities. It all remains a statistical balancing act of juggling potential risks versus benefits as we help patients navigate the cons/dangers posed by non-FDA-approved epidural spinal injections. Further, these unnecessary injections (i.e. well-documented minimal to no short-term (i.e. 3-6 weeks) and no long-term benefits) typically cost patients or their insurance carriers hundreds to over thousands of dollars per injection (i.e., varies by state, carrier, setting); certainly, these fees are lining pain management specialists’ pockets. Lastly, in some instances, epidural steroid injections are being wrongly performed, negligently delaying “essential” spine surgery.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
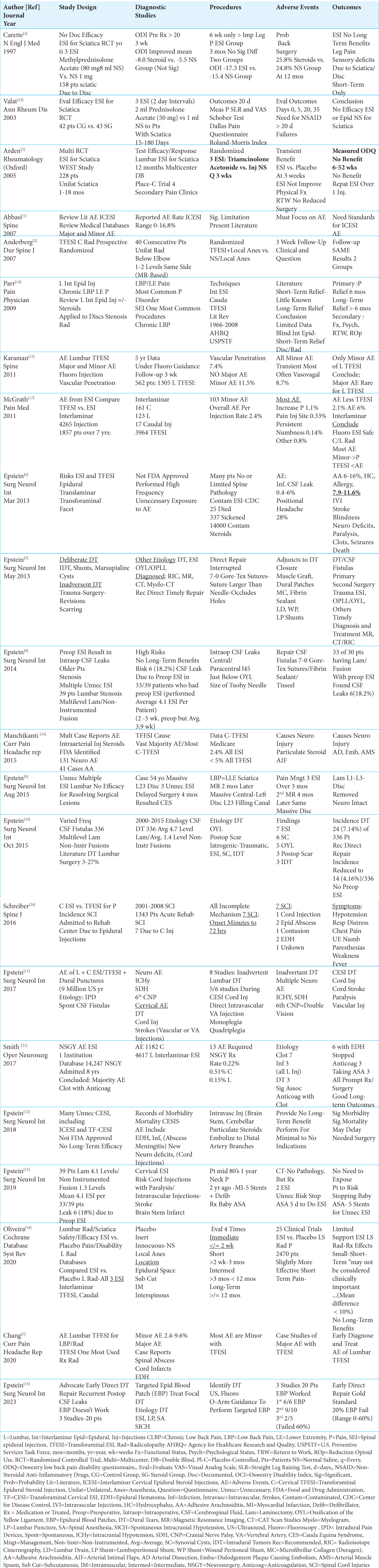
1. Abbasi A, Malhotra G, Malanga G, Elovic EP, Kahn S. Complications of interlaminar cervical epidural steroid injections: A review of the literature. Spine (Phila Pa 1976). 2007. 32: 2144-51
2. Anderberg L, Annertz M, Persson L, Brandt L, Saveland H. Transforaminal steroid injections for the treatment of cervical radiculopathy: A prospective and randomized study. Eur Spine J. 2007. 16: 321-8
3. Arden NK, Price C, Reading I, Stubbing J, Hazelgrove J, Dunne C. A multicenter randomized controlled trial of epidural corticosteroid injections for sciatica: the WEST study. Rheumatology (Oxford). 2005. 44: 1399-406
4. Carette S, Leclaire R, Marcoux S, Morin F, Blaise GA, St-Pierre A. Epidural corticosteroid injections for sciatica due to herniated nucleus pulposus. N Engl J Med. 1997. 336: 1634-40
5. Chang A, Ng AT. Complications associated with lumbar transforaminal epidural steroid injections. Curr Pain Headache Rep. 2020. 24: 67
6. Epstein NE. The risks of epidural and transforaminal steroid injections in the Spine: Commentary and a comprehensive review of the literature. Surg Neurol Int. 2013. 4: S74-93
7. Epstein NE. A review article on the diagnosis and treatment of cerebrospinal fluid fistulas and Dural tears occurring during spinal surgery. Surg Neurol Int. 2013. 4: S301-17
8. Epstein NE. Commentary: Unnecessary preoperative epidural steroid injections lead to cerebrospinal fluid leaks confirmed during spinal stenosis surgery. Surg Neurol Int. 2014. 5: S325-8
9. Epstein NE. Unnecessary multiple epidural steroid injections delay surgery for massive lumbar disc: Case discussion and review. Surg Neurol Int. 2015. 6: S383-7
10. Epstein NE. Incidence and management of cerebrospinal fluid fistulas in 336 multilevel laminectomies with noninstrumented fusions. Surg Neurol Int. 2015. 6: S463-8
11. Epstein NE. Neurological complications of lumbar and cervical Dural punctures with a focus on epidural injections. Surg Neurol Int. 2017. 8: 60
12. Epstein NE. Major risks and complications of cervical epidural steroid injections: An updated review. Surg Neurol Int. 2018. 9: 86
13. Epstein NE. Unnecessary cervical epidural injection in an octogenarian. Surg Neurol Int. 2019. 10: 108
14. Epstein NE, Agulnick MA. Perspective: Early direct repair of recurrent postoperative cerebrospinal (CSF) fluid leaks: No good evidence epidural blood patches (EBP) work. Surg Neurol Int. 2023. 14: 120
15. Karaman H, Kavak GO, Tufek A, Yldrm ZB. The complications of transforaminal lumbar epidural steroid injections. Spine (Phila Pa 1976). 2011. 36: E819-24
16. Manchikanti L, Hirsch JA. Neurological complications associated with epidural steroid injections. Curr Pain Headache Rep. 2015. 19: 482
17. McGrath JM, Schaefer MP, Malkamaki DM. Incidence and characteristics of complications from epidural steroid injections. Pain Med. 2011. 12: 726-31
18. Oliveira CB, Maher CG, Ferreira M, Hancock MJ, Oliveira VC, McLachlan A. Epidural corticosteroid injections for lumbosacral radicular pain. Cochrane Database Syst Rev. 2020. 4: CD013577
19. Parr AT, Diwan S, Abdi S. Lumbar interlaminar epidural injections in managing chronic low back and lower extremity pain: A systematic review. Pain Phys. 2009. 12: 163-88
20. Schreiber AL, McDonald BP, Kia F, Fried GW. Cervical epidural steroid injections and spinal cord injuries. Spine J. 2016. 16: 1163-6
21. Smith GA, Pace J, Strohl M, Kauo A, Hayek S, Miller JP. Rare neurosurgical complications of epidural injections: An 8-yr single-institution experience. Oper Neurosurg (Hagerstown). 2017. 13: 271-9
22. Valat JP, Giraudeau B, Rozenberg S, Goupille P, Bourgeois P, Micheau-Beaugendre V. Epidural corticosteroid injections for sciatica: A randomized, double blind, controlled clinical trial. Ann Rheum Dis. 2003. 62: 639-43






Jorge Luis Mercado Geney
Posted September 22, 2024, 5:24 am
Muy buen aporte, se está haciendo con mucha frecuencia sin resultados