- Division of Neurosurgery, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, United States
- Instituto Israelita de Ensino e Pesquisa, Hospital Israelita Albert Einstein, São Paulo, Brazil
- Mechanical Engineering Department, Maua Institute of Technology, São Paulo, Brazil
- Interventional Neuroradiology, Hospital Israelita Albert Einstein, São Paulo, Brazil
Correspondence Address:
Rafael Trindade Tatit, Instituto Israelita de Ensino e Pesquisa, Hospital Israelita Albert Einstein, São Paulo, Brazil.
DOI:10.25259/SNI_686_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Christopher S. Ogilvy1, Rafael Trindade Tatit2, Vincenzo T. R. Loly2, Felipe Ramirez-Velandia1, João S. B. Lima3, Carlos E. Baccin4. Exploring the hemodynamic behavior of residual aneurysms after coiling and clipping: A computational flow dynamic analysis. 18-Oct-2024;15:376
How to cite this URL: Christopher S. Ogilvy1, Rafael Trindade Tatit2, Vincenzo T. R. Loly2, Felipe Ramirez-Velandia1, João S. B. Lima3, Carlos E. Baccin4. Exploring the hemodynamic behavior of residual aneurysms after coiling and clipping: A computational flow dynamic analysis. 18-Oct-2024;15:376. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13153
Abstract
Background: Residual intracranial aneurysms post-clipping or coiling pose a poorly established risk of rupture. Computational fluid dynamic (CFD) offers insights into hemodynamic changes following such interventions. This study aims to assess hemodynamic parameters in residual aneurysms pre- and post-treatment with surgical clips or coils using CFD.
Methods: A retrospective analysis of consecutive patients between January 2015 and January 2024 was conducted. Digital subtraction angiography images were reconstructed using 3D modeling techniques, and hemodynamic parameters were analyzed with ANSYS® software.
Results: Six aneurysms were analyzed: Five unruptured and one ruptured. The aneurysms were located at the basilar apex (2), middle cerebral artery bifurcation (2), and origin of the posterior communicating artery (2). Post-treatment, there was a significant reduction in both aneurysm area (median reduction of 33.73%) and volume (median reduction of 25.3%). Five of the six cases demonstrated fewer low wall shear stress (WSS) areas, which could indicate a reduction in regions prone to thrombus formation and diminished risk of rupture. In the unruptured aneurysms, there was a median increase of 137.6% in average WSS. Notably, the only case with increased low WSS area also had the highest increase in average WSS. One basilar artery aneurysm showed increased WSS across all parameters, suggesting a higher rupture risk.
Conclusion: The increase in average and high WSS area, along with a decrease in low WSS area, reflects a complex balance between factors of stability and rupture risk. However, a simultaneous increase in all WSS parameters may represent the highest rupture risk due to increased mechanical stress on the aneurysm wall, necessitating closer monitoring.
Keywords: Computational fluid dynamics, Low shear area, Residual intracranial aneurysms, Wall shear stress
INTRODUCTION
Background
Intracranial residual intracranial aneurysms (IAs) are those with angiographic evidence of persistent filling after treatment. The frequency of incomplete aneurysm occlusion after surgical clipping varies from 4.7% to 23%[
Given the elevated prevalence of residual aneurysms and the potentially heightened risk of rupture in these instances, there is a considerable need for the development of tools and the establishment of adjunct parameters in stratifying the risk of these residual aneurysms. In this context, computational fluid dynamics (CFD) appears to offer insights into hemodynamic factors that may influence the rupture of these aneurysms. A meta-analysis suggested that decreased local wall shear stress (WSS) may be an important predictive parameter of aneurysm rupture.[
Objectives
Herein, we assess hemodynamic parameters before and after treatment with surgical clips or coils on residual IAs using CFD. Insights into the most affected parameters by the respective treatments will be derived.
MATERIALS AND METHODS
This study was reviewed and approved by the institutional ethics committee. Informed consent was obtained from all patients or their next of kin.
Patient data
We conducted a retrospective investigation of data from consecutive patients treated between January 2015 and January 2024 for both ruptured and unruptured IAs, either by surgical clipping or coil embolization, with or without associated stenting. These patients exhibited residual aneurysms or necks on follow-up digital subtraction angiography (DSA). All digital angiography images of selected patients, both pre-and post-treatment, were anonymized.
Geometry reconstruction
For aneurysm geometry reconstruction, selected digital angiography examinations were processed using the 3D Slicer package, a software designed for 3D reconstruction and geometric analysis of vascular image-based modeling. From these images, stereolithography (STL) segmentations were created and subsequently imported into the SpaceClaim computer-aided design software for geometry processing. Following preparation, the mesh generation step was carried out using Ansys Fluent Meshing. Reconstructed geometries were compared with patient images and discussed with an interventional neuroradiologist for model approval.
Hemodynamic models
The numerical simulations were carried out in the software Fluent, part of the ANSYS® package. To handle fluid-structure interaction, we employed a partitioned coupling scheme specifically developed for incompressible flows interacting with solids subject to elastic or plastic deformations, as observed in arteries.
To estimate the average flow rate of each artery, we relied on the study by Zarrinkoob et al.,[
Hemodynamic parameters
We analyzed the volume, surface area, and WSS before and after aneurysm treatment, as well as WSS in adjacent arteries. From flow velocity and pressure fields, we derived stress and strain fields of artery and aneurysm walls. These fields were used to calculate parameters such as WSS and their derivatives, as well as the magnitude of wall deformations caused by velocity and pressure fields. Similarly to the study by Hu et al.,[
RESULTS
Aneurysm characteristics
Six patients with single cerebral aneurysms were included in this study, with pre-and post-treatment DSA images available for analysis. Regarding the aneurysm locations, two were in the middle cerebral artery (MCA), two at the apex of the basilar artery, and two in the posterior communicating artery (PComA) with the internal carotid artery (ICA). Among these six identified aneurysms, five were treated with coil embolization and one with surgical clipping, situated in the MCA. Regarding rupture status, only one case was ruptured before treatment, located at the apex of the basilar artery.
Hemodynamic parameters in the pre-clipping and coil model
Two unruptured aneurysms at the bifurcation of the MCA were evaluated: one was treated with a surgical clip, and the other with endovascular coils and a Y-stent (Neuroform EZ). The surgical case aneurysm dome area was 60.65 mm2, volume 48.78 mm3, average WSS 14.28 Pa, and low WSS area 0.2%. The endovascular case aneurysm dome area was 215.88 mm2, volume 289.85 mm3, average WSS 2.44 Pa, and low WSS area 7.49%.
Two unruptured aneurysms at the bifurcation of the PComA with the ICA were treated with coils. One of the aneurysms had a dome area of 279.02 mm2, a volume of 379.28 mm3, an average WSS of 0.35 Pa, and a low WSS area of 74.9%. Another aneurysm had a dome area of 181.29 mm2, volume of 208.73 mm3, average WSS of 1.41 Pa, and low WSS area of 55.8%.
Two aneurysms at the apex of the basilar artery were treated endovascularly, one unruptured and another ruptured. The unruptured case was treated with coils and a stent (Neuroform Atlas) left under the P1 segment of the posterior cerebral artery (PCA). The aneurysm dome area was 207.47 mm2, volume 306.97 mm3, average WSS 2.41 Pa, and low WSS area 1.38%. The ruptured case was treated only with coils. The aneurysm dome area was 72.3 mm2, volume 51.99 mm3, average WSS 8.31 Pa, and low WSS area 21.7%.
Comparing pre- and post-clipping and coil model
After treatment, there was a reduction in both the area (median 33.74%; interquartile range [IQR] = 18.83%) and volume (median 19.37%; IQR = 23.43%) of all six identified aneurysms. Only one of the aneurysms was ruptured at the initial presentation. Among the cases analyzed, five exhibited increases in average WSS after treatment (median increase of 137.6%; IQR = 136.9%), five demonstrated a reduction in the low WSS area (median decrease of 70%; IQR = 49.72%), and five cases displayed an increase in high WSS area (median increase of 317.9%; IQR = 364.14%). When considering the location, it is notable that the two aneurysms located at the bifurcation of the MCA exhibited a reduction in the low WSS area (0.2–0.006% and 7.49–3.57%), coupled with an elevation in the average WSS (increase of 23.67% and 94.71%). Notably, among these, the residual aneurysm displayed a comparatively minor reduction in size, with its remaining portion constituting 58.61% of the original total volume, and also demonstrated a decrease in the high WSS area (99.1–94.6%). Regarding the two cases identified at the origin of the PComA, both demonstrated similar behavior of increased average WSS (an increase of 191.43% and 137.59%) and high WSS area (0.5–4.87% and 5.2–9.11%) and decreased low WSS area (74.9–66.7% and 55.8–4.98%). Finally, two cases at the basilar apex exhibited markedly different behaviors. Although both experienced an increase in the high WSS area (6.23–33.6% and 49.2–50.6%), the ruptured aneurysm demonstrated decreases in both average WSS (decrease 9.96%) and low WSS area (21.7–6.15%), whereas the unruptured case demonstrated increases in both average WSS (increase 200.83%) and low WSS area (1.38–7.82%). Notably, this unique case with an increase in low WSS area was the one with the highest increase in average WSS, both in relative (an increase of 200.83%) and absolute terms (an increase of 4.84 Pa). A detailed description of the hemodynamic parameters of each of the residual aneurysms and changes after treatment is shown in
Long-term outcomes of conservative and reintervention strategies
Both MCA residual aneurysms were managed conservatively, remaining without bleeding for 7 and 9 years up to the present date. On the other hand, both PComA-ICA residual aneurysms underwent successful reintervention with flow diverter stents, achieving complete occlusion until the last follow-up, 7 months after the reinterventions. Finally, the basilar artery apex residual aneurysm that had previously ruptured at the initial presentation remained stable without new hemorrhagic events for 3 years up to this moment. On the other hand, the other basilar artery apex residual aneurysm was retreated with the placement of another stent and coils, creating a Y-stenting, achieving complete occlusion until the last follow-up, 6 months post-reintervention.
Illustrative case (MCA)
A 62-year-old female experienced subarachnoid hemorrhage due to the rupture of an aneurysm in the ICA. The ruptured aneurysm was treated endovascularly with coils, achieving complete obliteration. Incidentally, another aneurysm was identified in the MCA, which was later treated surgically. However, post-surgical digital angiography revealed a residual portion of the clipped aneurysm. Following surgery, this residual aneurysm was evaluated using CFD, comparing its morphology and hemodynamics to its pre-treatment state [
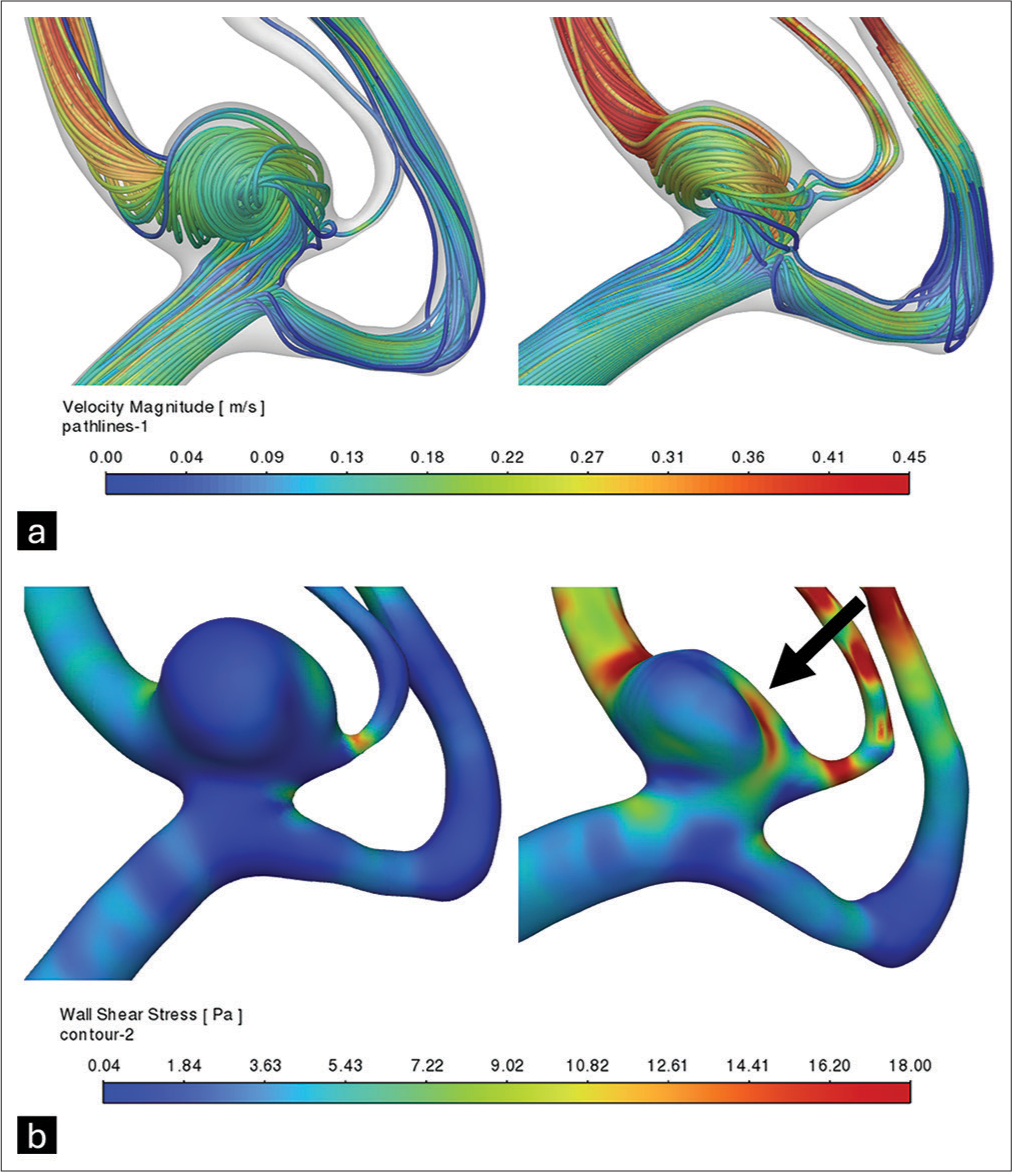
Figure 1:
Reconstruction and hemodynamic analysis of the middle cerebral artery bifurcation aneurysm before and after clipping. (a) Estimated blood velocity along the aneurysm and adjacent arteries. (b) Estimated wall shear stress on the aneurysm and adjacent arterial walls, demonstrating elevated wall shear stress adjacent to the surgical clip site (arrow).
The residual volume was calculated at 58.61% of the initial volume (48.78–28.59 mm3), and although the average WSS increased by 23.67% (+3.38 Pa), the analysis showed a decrease in the low WSS area (0.2–0.006%) and high WSS area (99.1–94.6%).
Illustrative case (PComA)
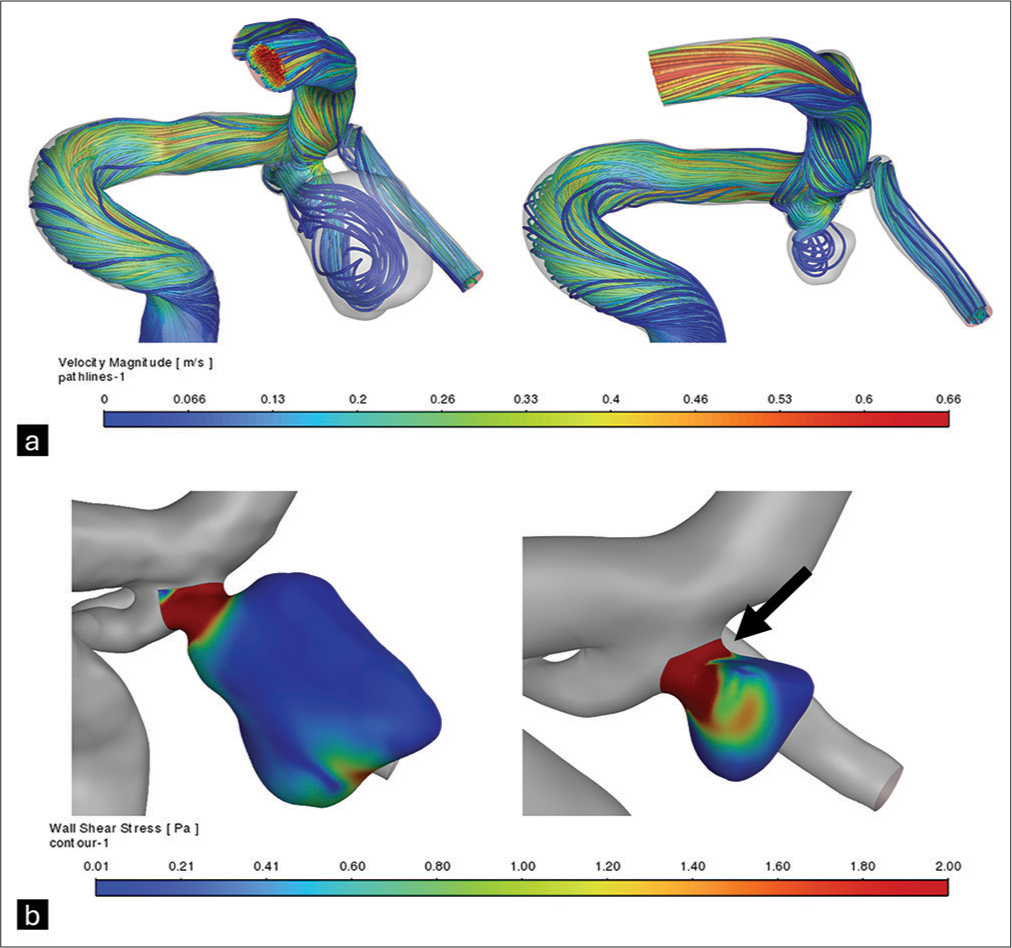
A 63-year-old male presenting with mental confusion and undergoing investigation for intestinal neoplasia was found to have an aneurysm at the bifurcation of the PComA with the ICA on magnetic resonance imaging (MRI), along with adjacent temporal lobe edema. To protect the aneurysm dome, partial embolization was performed before gastrointestinal oncologic surgery. The protective embolization reduced the aneurysm to 9.35% of its initial volume and showed increased average WSS (+191.43%) and high WSS area (0.5–4.87%), along with decreased low WSS area (74.9–66.7%) in the hemodynamic analysis [
Figure 2:
Reconstruction and hemodynamic analysis of the posterior communicating artery-internal carotid artery bifurcation aneurysm before and after coiling. (a) Estimated blood velocity along the aneurysm and adjacent arteries. (b) Estimated wall shear stress on the walls of the aneurysm, demonstrating an increase in wall shear stress near the aneurysm neck region (arrow).
Subsequently, a flow-diverter was deployed, resulting in a complete occlusion of the aneurysm at the latest angiographic follow-up [
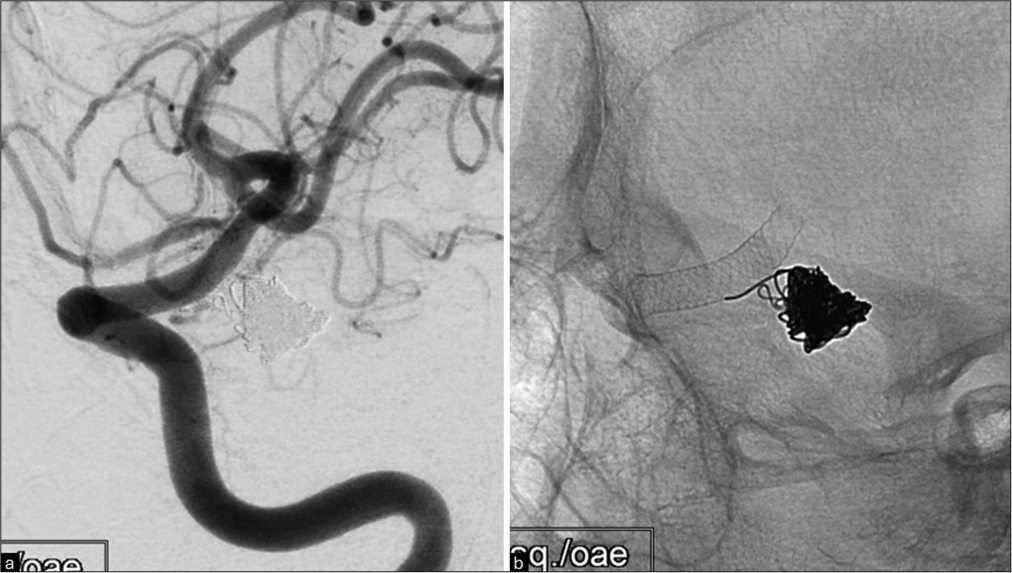
Figure 3:
Post-reintervention images of the posterior communicating artery-internal carotid artery bifurcation aneurysm. (a) Left internal carotid artery injection oblique view follow-up showing complete occlusion of the left posterior communicating artery aneurysm post-endovascular treatment. (b) Plain X-ray showing the flow diverter stent and coils placed inside the aneurysm sac.
Illustrative case (basilar artery apex)
A 63-year-old female was diagnosed with an unruptured aneurysm at the apex of the basilar artery following an MRI scan in a workup for dizziness. Endovascular embolization was performed using coils and a stent (Neuroform Atlas) placed under the P1 segment of the left PCA, originating from the basilar artery. Following this intervention, the aneurysm showed a residual volume of 12.07% of the initial size, accompanied by increases in average WSS (+200.83%); [
Figure 4:
Reconstruction and hemodynamic analysis of the basilar artery apex aneurysm before and after single stent (from left P1 to basilar artery). (a) Velocity streamlines indicate inflow predilection to the right side of the aneurysm wall before treatment and interruption of the main streamlines due to coils after coil embolization. (b) Reduction in aneurysm volume after coil embolization, with residual aneurysm showing increased wall shear stress, partially visualized in this incidence near the neck (arrow).
Given these hemodynamic results indicating an increased risk of rupture, a decision was made for re-intervention by placing an additional stent in the P1 segment of the right PCA to the basilar artery, configuring a Y-stent, along with additional coil embolization [
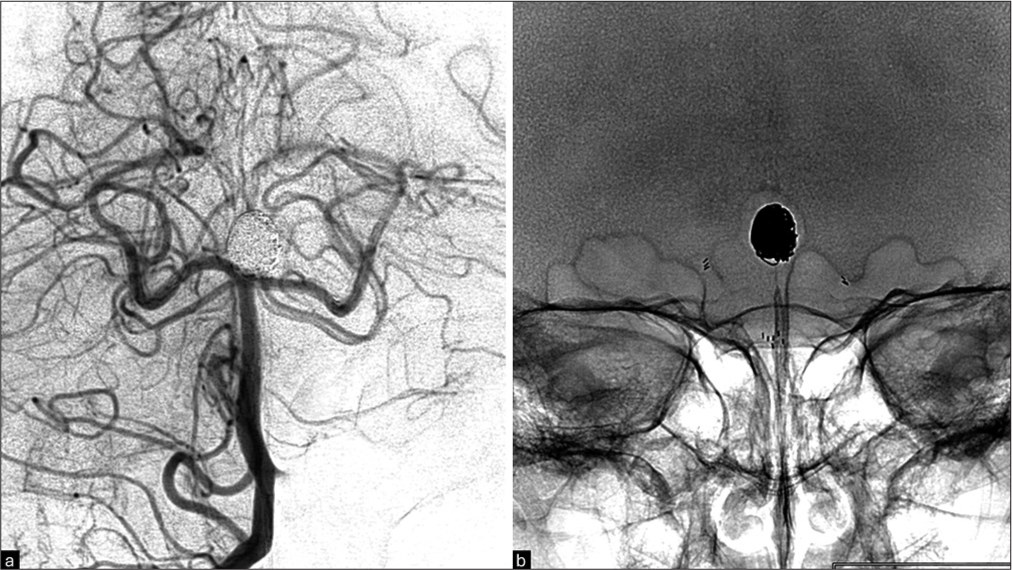
Figure 6:
Post-reintervention images of the basilar artery apex aneurysm, demonstrating complete occlusion after retreatment with additional stent (Y-stenting) and coils. (a) Basilar digital subtraction angiography with right vertebral artery selective angiography. (b) Plain X-ray showing the Y-stenting and coils.
DISCUSSION
Key findings
In this computational flow dynamics analysis, we observed a consistent reduction in both the area (median 33.74%; IQR = 18.83%) and volume (median 19.37%; IQR = 23.43%) across all six residual aneurysms. Furthermore, we noted significant variability in the alteration patterns of average WSS, regions with high and low WSS, and changes in flow velocities within terminal branches. Of note, the prevalent trend among the observed changes was an augmentation in average WSS (median increase of 137.6%; IQR = 59.2–196.1) coupled with a concurrent decrease in low WSS areas. This trend underscores the potential utility of segmental analysis in assessing surface area variations of aneurysms and understanding the implications of low WSS regions on rupture risk. Among the six cases examined, one exhibited a distinct pattern characterized by an increase in average WSS alongside elevated areas of low WSS. This particular case, we posit, presents the highest likelihood of rupture and warrants closer surveillance.
Interpretation
To gauge the rupture risk post-treatment for aneurysms that had ruptured before initial intervention (i.e., with subarachnoid hemorrhage), a noteworthy study by Johnston et al.[
Out of our six studied cases, only one of the aneurysms was ruptured at the initial presentation. Past cohorts have evaluated the risk of rupture for residual aneurysms according to the aneurysm rupture status, especially in cases treated with coils. In an insightful study by Munich et al.,[
In our results, the decreasing trend of the low WSS areas in most cases (five of the six cases) could indicate a reduction in regions prone to thrombus formation[
Notably, the only case with an increase in low WSS area was the one with the highest increase in average WSS, both in relative (+200.83%) and absolute terms (+4.84 Pa). This case, with an increase in low WSS area and the highest increase in WSS, may represent a higher-risk scenario due to the potential for residual thrombus formation and greater mechanical stress on the aneurysm wall, which could lead to instability and necessitate closer monitoring.[
This result underscores the intricate relationship between hemodynamic parameters and the risk of cerebral aneurysm rupture or stability post-intervention. While the pre-treatment unruptured state and significant reductions in aneurysm size imply a lower risk of rupture, the interplay between increased WSS and increased low WSS area highlights the need for careful post-treatment monitoring to avoid complications.
Limitations and generalizability
Our study, although informative, has several limitations that must be considered. First, the retrospective design and small sample size of six patients may limit the generalizability of our findings. In addition, the use of CFD introduces inherent assumptions and simplifications that could affect the accuracy of our results. Furthermore, while we focused on key hemodynamic parameters such as WSS and low WSS area, other factors influencing treatment outcomes were not explored. Finally, interpreting hemodynamic changes post-intervention requires consideration of various clinical and imaging factors. Thus, while our study provides valuable insights, further research with larger samples and comprehensive approaches is warranted to validate and extend our findings.
CONCLUSION
Residual IAs display diverse hemodynamic responses following clipping or coiling procedures. The observed increase in high WSS area and average WSS, coupled with a decrease in low WSS area, suggests a complex interaction between stability and rupture risk factors. Conversely, an increase of all parameters may indicate the riskiest scenario due to greater mechanical stress on the aneurysm wall, potentially leading to instability and rupture and requiring closer monitoring.
Ethical approval
The research/study is approved by the Institutional Review Board at Hospital Israelita Albert Einstein, number 6.713.150, dated March 20, 2024.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Aguiar GB, Kormanski MK, Corrêa CJ, Batista AV, Conti ML, Veiga JC. Residual lesions in patients undergoing microsurgical clipping of cerebral aneurysms in a reference university hospital. Clinics. 2020. 75: e1973
2. Bass DI, Marsh LM, Fillingham P, Lim D, Chivukula VK, Kim LJ. Modeling the mechanical microenvironment of coiled cerebral aneurysms. J Biomech Eng. 2023. 145: 041005
3. Bederson JB, Awad IA, Wiebers DO, Piepgras D, Haley EC, Brott T. Recommendations for the management of patients with unruptured intracranial aneurysms. Circulation. 2000. 102: 2300-8
4. Dolan JM, Kolega J, Meng H. High wall shear stress and spatial gradients in vascular pathology: A review. Ann Biomed Eng. 2013. 41: 1411-27
5. Ford MD, Alperin N, Lee SH, Holdsworth DW, Steinman DA. Characterization of volumetric flow rate waveforms in the normal internal carotid and vertebral arteries. Physiol Meas. 2005. 26: 477-88
6. Hu S, Chen R, Xu W, Li H, Yu J. A predictive hemodynamic model based on risk factors for ruptured mirror aneurysms. Front Neurol. 2022. 13: 998557
7. Johnston SC, Dowd CF, Higashida RT, Lawton MT, Duckwiler GR, Gress DR. Predictors of rehemorrhage after treatment of ruptured intracranial aneurysms. Stroke. 2008. 39: 120-5
8. Jou LD, Lee DH, Morsi H, Mawad ME. Wall shear stress on ruptured and unruptured intracranial aneurysms at the internal carotid artery. Am J Neuroradiol. 2008. 29: 1761-7
9. Munich SA, Cress MC, Rangel-Castilla L, Sonig A, Ogilvy CS, Lanzino G. Neck remnants and the risk of aneurysm rupture after endovascular treatment with coiling or stent-assisted coiling: Much ado about nothing?. Neurosurgery. 2019. 84: 421-7
10. Murias Quintana E, Gil Garcia A, Vega Valdés P, Cuellar H, Meilán Martínez Á, Saiz Ayala A. Anatomical results, rebleeding and factors that affect the degree of occlusion in ruptured cerebral aneurysms after endovascular therapy. J Neurointerv Surg. 2015. 7: 892-7
11. Obermueller K, Hostettler I, Wagner A, Boeckh-Behrens T, Zimmer C, Gempt J. Frequency and risk factors for postoperative aneurysm residual after microsurgical clipping. Acta Neurochir (Wien). 2021. 163: 131-8
12. Qiu T, Jin G, Xing H, Lu H. Association between hemodynamics, morphology, and rupture risk of intracranial aneurysms: A computational fluid modeling study. Neurol Sci. 2017. 38: 1009-18
13. Rayz VL, Boussel L, Lawton MT, Acevedo-Bolton G, Ge L, Young WL. Numerical modeling of the flow in intracranial aneurysms: Prediction of regions prone to thrombus formation. Ann Biomed Eng. 2008. 36: 1793-804
14. Sauvigny J, Drexler R, Pantel TF, Ricklefs FL, Catapano JS, Wanebo JE. Microsurgical clipping of unruptured anterior circulation aneurysms-a global multicenter investigation of perioperative outcomes. Neurosurgery. 2024. 94: 1218-26
15. Shojima M, Oshima M, Takagi K, Torii R, Hayakawa M, Katada K. Magnitude and role of wall shear stress on cerebral aneurysm. Stroke. 2004. 35: 2500-5
16. Spiessberger A, Vogt DR, Fandino J, Marbacher S. Formation of intracranial de novo aneurysms and recurrence after neck clipping: A systematic review and meta-analysis. J Neurosurg. 2020. 132: 456-64
17. Tian Z, Li X, Wang C, Feng X, Sun K, Tu Y. Association between aneurysmal hemodynamics and rupture risk of unruptured intracranial aneurysms. Front Neurol. 2022. 13: 818335
18. Tsutsumi K, Ueki K, Usui M, Kwak S, Kirino T. Risk of recurrent subarachnoid hemorrhage after complete obliteration of cerebral aneurysms. Stroke. 1998. 29: 2511-3
19. Wang JW, Li CH, Liu JF, Li H, Guo H, Gao BL. Endovascular treatment of multiple intracranial aneurysms. Medicine. 2023. 102: e36340
20. Wermer MJ, Rinkel GJ, Greebe P, Albrecht KW, Dirven CM, Tulleken CA. Late recurrence of subarachnoid hemorrhage after treatment for ruptured aneurysms: Patient characteristics and outcomes. Neurosurgery. 2005. 56: 197-204
21. Zarrinkoob L, Ambarki K, Wåhlin A, Birgander R, Eklund A, Malm J. Blood flow distribution in cerebral arteries. J Cereb Blood Flow Metab. 2015. 35: 648-54
22. Zhou G, Zhu Y, Yin Y, Su M, Li M. Association of wall shear stress with intracranial aneurysm rupture: Systematic review and meta-analysis. Sci Rep. 2017. 7: 5331