- Department of Neurosurgery, National Institute of Neurology and Neurosurgery, Tlalpan, Mexico
- Department of Neurosurgery, Hospital Zambrano Hellion, Tecnologico de Monterrey, San Pedro Garza García, Mexico
- Department of Spine, National Institute of Neurology and Neurosurgery, Tlalpan, Mexico
Correspondence Address:
Victor Alcocer-Barradas, Department of Neurosurgery, National Institute of Neurology and Neurosurgery, Tlalpan, Mexico.
DOI:10.25259/SNI_323_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Marco Antonio Munuzuri-Camacho1, Ricardo Palacios-Rodriguez1, Jorge Alanis-Mendizabal1, Tomas Moncada-Habib1, Marcos V. Sangrador-Deitos2, Obet Jair Canela-Calderon3, Victor Alcocer-Barradas1. Combined microscopic transoral and endoscopic endonasal approach for a clival chordoma: A case report and literature review. 25-Oct-2024;15:383
How to cite this URL: Marco Antonio Munuzuri-Camacho1, Ricardo Palacios-Rodriguez1, Jorge Alanis-Mendizabal1, Tomas Moncada-Habib1, Marcos V. Sangrador-Deitos2, Obet Jair Canela-Calderon3, Victor Alcocer-Barradas1. Combined microscopic transoral and endoscopic endonasal approach for a clival chordoma: A case report and literature review. 25-Oct-2024;15:383. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13169
Abstract
Background:Chordomas are primary bone tumors derived from the embryonic notochord. They represent 1–4% of all malignant bone tumors. They have a predominantly extra-axial location, arising in the clival region in 35% of reported cases. The prognosis is generally poor, and radical resection remains the first-line treatment. This study aims to describe a case of a clival chordoma that was resected through a combined microscopic transoral and endoscopic endonasal approach, with excellent clinical outcomes.
Case Description:A 24-year-old woman with low cranial nerve symptomatology was admitted for a two-stage surgical approach. An occipital-cervical fixation was performed in the first stage, while a combined endonasaltransoral resection was performed later for tumor resection.
Conclusion:Microscopic transoral and endoscopic endonasal approaches offer advantages for treating clival chordomas, with careful consideration of anatomical constraints and potential for postoperative recurrence being essential in approach selection.
Keywords: Chordoma, Clivus, Endoscopic endonasal approach, Microscopic transoral approach
INTRODUCTION
Chordomas are primary bone tumors derived from the embryonic notochord[
Clinically, superior clival lesions may affect the upper cranial nerves, mainly CN VI,[
The prognosis is generally poor, and radical resection remains the first-line treatment. Although the use of adjuvant radiation therapy remains controversial, extensive resection followed by high-dose radiation therapy offers the best chance of achieving long-term tumor control[
There are multiple surgical approaches for clival chordoma resection, and classically, the transcranial route has been the preferred method; nevertheless, with the advent of endoscopic techniques, the transoral and transnasal routes are deemed effective for resection, depending on the extension, with the possibility to encompass all the clivus from the posterior clinoids to the craniovertebral junction (CVJ).[
Endoscopic endonasal approaches (EEAs) provide better visualization and lower postoperative complication rates than open approaches do, except for cerebrospinal fluid (CSF) leak rates.[
On the other hand, microscopic transoral approaches (MTAs) are used for midline ventral lesions ranging from the lower third of the clivus to the C2 vertebral body, including a complete view of the vertebrobasilar arterial system and cranial nerves III–XII.[
Both techniques provide direct access to the CVJ. The EEA has the advantage of being a shorter route, while the MTA provides a wider surgical field.[
This study aims to describe the case of a clival chordoma, a brief review of the literature, and a technical note on surgical resection using a combined MTA and EEA.
ILLUSTRATIVE CASE
A 24-year-old woman with no relevant medical history presented as an outpatient with rhinorrhea, foreign body sensation in the oropharynx, dysphagia, cervicalgia, and tinnitus. Medical records revealed a previous transoral biopsy that had already diagnosed the condition as chordoma, necessitating a neurosurgical consultation. Physical examination revealed malnourishment due to dysphagia, nasal obstruction requiring mouth-breathing, and secondary xerosis. No motor or sensory alterations in cranial nerves were found. Magnetic resonance imaging (MRI) and computed tomography (CT)-angiography were performed [
Operative technique
First stage: Occipitocervical fixation
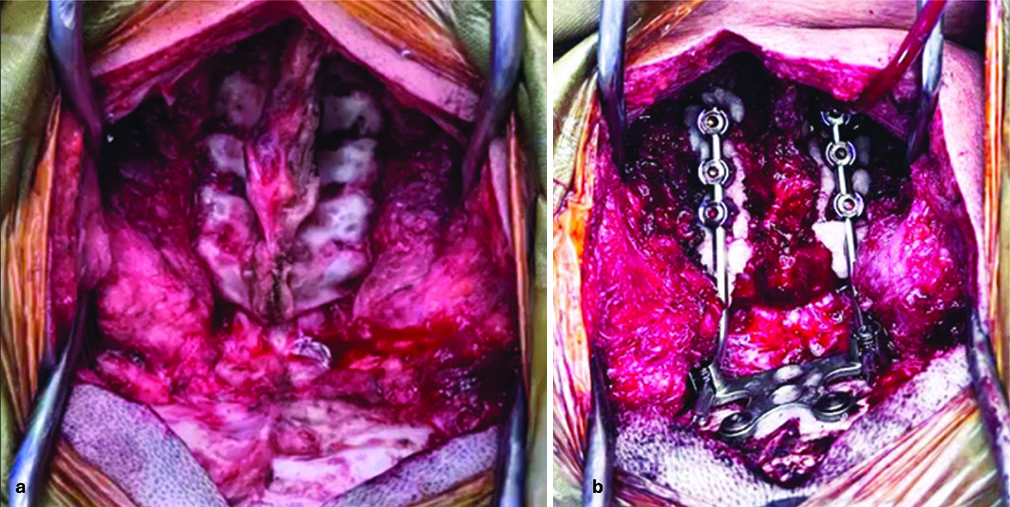
With the patient in a prone decubitus position and the head fixed in a Mayfield-Kees head holder, a midline suboccipital incision extending to the spinous process of C6 was performed. Dissection was carried out through soft-tissue planes, performing subperiosteal dissection to expose the CVJ, extending down to C6, exposing its lateral masses. An occipital plate was then positioned beneath the projection of the transverse sinus and the confluence of the sinuses. Transpedicular screws were inserted at C3, C5, and C6 (12 mm, 14 mm, and 14 mm, respectively), followed by fixation with 10 cm titanium rods [
Second stage: Combined microscopic transoral and endoscopic endonasal resection
With the patient positioned supine and the head in a neutral position, 3-point skeletal fixation was applied using a Mayfield-Kees head holder. Oxymetazoline-soaked cottonoids were inserted into both nostrils for 5 min. Subsequently, with a 0° rigid endoscope, the right middle and inferior turbinates were laterally displaced. An incision was made at the mucocutaneous junction of the septum, followed by dissection of the cartilaginous portion in the subperichondrial plane, and a posterior septectomy was performed to access the rostral side of the sphenoid rostrum. Right maxillary uncinectomy was performed, and an ipsilateral pedicled nasoseptal flap was harvested. The sphenoid sinus ostia were identified, and the rostrum was removed with the assistance of a 4 mm diamond burr. Once inside the sphenoid sinus, identification of the sellar region and the clival recess was achieved through drilling and blunt dissection. The tumor was located along the most ventral portion of the clival recess. Resection was initiated using bipolar coagulation, ringed curettes, and a conventional aspirator, as no endonasal attachments for the ultrasonic aspirator were available. The approach was widened by reaming the floor of the sella turcica and the clivus down to its inferior portion, where erosion was observed. Subtotal resection was deemed safe and we decided not to violate the dural plane, as by doing this, a high-flow CSF fistula would certainly arise. This could lead to serious postoperative complications, and as only a very small portion of the lesion was located intradurally (<5% of the total volume), no changes in overall survival would have been achieved. Subsequently, the transoral approach was performed using open microsurgical techniques. A mouth retractor was placed, and the uvula with the soft palate was retracted dorsally using 1-0 silk. A scalpel incision was made in the posterior pharyngeal wall at the level of the anterior arch of C1, followed by deepening over soft tissues with monopolar electrocautery until reaching the tumor. Resection continued with the assistance of an ultrasonic aspirator, with no complications. Tumor consistency was heterogeneous, with soft areas alternating with very hard portions that could be hardly devastated, even with the ultrasonic aspirator. On completion of the resection, reconstruction of the approach was performed. At the transoral level, the pharyngeal mucosa was closed with 2-0 Vicryl sutures. At the endonasal level, hemostasis of the surgical bed was ensured with Surgicel fragments, followed by placement of the pedicled nasoseptal flap. Tissue adherents were applied to the surgical edges, and nasal packing was performed with two Foley tubes, inflating the upper balloon with 7 mL and the lower one with 6 mL. The procedure was then concluded [
Video 1
Follow-up
There were no postoperative complications and the patient was dispatched after 8 days of hospitalization. After 3 months, the patient presented with clinical and radiological signs of hydrocephalus; thus, a ventriculoperitoneal shunt was placed. At follow-up as an outpatient no additional neurological deficits were found, however, tracheostomy and gastrostomy were left in place due to persistence of lower cranial nerve dysfunction. Postoperative CT-scan and MRI [
DISCUSSION
Occipitocervical fixation was first performed due to the involvement of the condyles and craniocervical junction (C1–C3), which posed a high risk of instability after resection. This fixation ensured structural stability and prevented complications. Once stabilized, a combined microscopic transoral and endoscopic endonasal approach was used for resection.
When we consider the advantages of these approaches (MTA/EEA), both have the advantage of providing an excellent extradural route without brain retraction.
Exposure from an MTA is limited by the degree of mouth opening and depends on the patient’s tongue size, position of the soft palate, and size of the oral cavity.[
The nasopalatine line described by Kassam predicts the inferior limit of the view in EEA.[
The postoperative outcomes of the EEA and MTA must be analyzed when selecting the procedure. The median length of hospital stay on the EEA in some series has been reported to be around 9 days, with a postoperative Karnofsky Performance Scale (KPSS) of 90% and recovery rates from cranial nerve palsy up to 75%.[
GTR is the desirable result when we operate on any brain tumor, but we need to consider the importance of the surrounding structures of each surgical field. Thus, a marginal resection should be considered as an acceptable result, taking into consideration that 10-year recurrence rates vary from 16% to 45%. Even when the macroscopic result seems to achieve GTR, most chordomas are likely to regrow.[
CONCLUSION
MTA and EEA offer significant advantages for the management of clival chordomas. While the EEA tends to displace fewer structures, the MTA provides adequate exposure to the lower third of the clivus. However, achieving GTR can be challenging due to anatomical limitations, and postoperative recurrence is a risk to consider. Selection of the surgical approach should be based on careful assessment of risks and benefits, as well as the specific anatomy of the patient and the lesion.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Video available on:
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Al-Mefty O, Borba LA. Skull base chordomas: A management challenge. J Neurosurg. 1997. 86: 182-9
2. Bai J, Li M, Shi J, Jing L, Zhai Y, Zhang S. Mid-term follow-up surgical results in 284 cases of clival chordomas: The risk factors for outcome and tumor recurrence. Neurosurg Rev. 2022. 45: 1451-62
3. Bai J, Li M, Xiong Y, Shen Y, Liu C, Zhao P. Endoscopic endonasal surgical strategy for skull base chordomas based on tumor growth directions: Surgical outcomes of 167 patients during 3 years. Front Oncol. 2021. 11: 724972
4. Baig Mirza A, Ravindran V, Okasha M, Boardman TM, Maratos E, Sinan B. Systematic review comparing open versus endoscopic surgery in clival chordomas and a 10-year single-center experience. J Neurol Surg B Skull Base. 2022. 83: e113-25
5. Bergh P, Kindblom LG, Gunterberg B, Remotti F, Ryd W, Meis-Kindblom JM. Prognostic factors in chordoma of the sacrum and mobile spine: A study of 39 patients. Cancer. 2000. 88: 2122-34
6. Boari N, Gagliardi F, Cavalli A, Gemma M, Ferrari L, Riva P. Skull base chordomas: Clinical outcome in a consecutive series of 45 patients with long-term follow-up and evaluation of clinical and biological prognostic factors. J Neurosurg. 2016. 125: 450-60
7. Butenschoen VM, Krauss P, Bernhardt D, Negwer C, Combs S, Meyer B. The transnasal endoscopic approach for resection of clival tumors: A single-center experience. Sci Rep. 2023. 13: 3012
8. Carpentier A, Polivka M, Blanquet A, Lot G, George B. Suboccipital and cervical chordomas: The value of aggressive treatment at first presentation of the disease. J Neurosurg. 2002. 97: 1070-7
9. Cavallo LM, Cappabianca P, Messina A, Esposito F, Stella L, de Divitiis E. The extended endoscopic endonasal approach to the clivus and cranio-vertebral junction: anatomical study. Childs Nerv Syst. 2007. 23: 665-71
10. Cho YH, Kim JH, Khang SK, Lee JK, Kim CJ. Chordomas and chondrosarcomas of the skull base: Comparative analysis of clinical results in 30 patients. Neurosurg Rev. 2008. 31: 35-43
11. Choi D, Crockard HA. Evolution of transoral surgery: Three decades of change in patients, pathologies, and indications. Neurosurgery. 2013. 73: 296-303 discussion 303-4
12. De Almeida JR, Zanation AM, Snyderman CH, Carrau RL, Prevedello DM, Gardner PA. Defining the nasopalatine line: The limit for endonasal surgery of the spine. Laryngoscope. 2009. 119: 239-44
13. De Divitiis O, Conti A, Angileri FF, Cardali S, La Torre D, Tschabitscher M. Endoscopic transoral-transclival approach to the brainstem and surrounding cisternal space: Anatomic study. Neurosurgery. 2004. 54: 125-30
14. Dusick JR, Esposito F, Kelly DF, Cohan P, DeSalles A, Becker DP. The extended direct endonasal transsphenoidal approach for nonadenomatous suprasellar tumors. J Neurosurg. 2005. 102: 832-41
15. Kirollos RW, Pillay R. Time to modify rather than discard the transoral approach to selected cases of Clival Chordomas at the craniocervical junction. Indian J Neurosurg. 2023. 12: 10-4
16. Lanzino G, Dumont AS, Lopes MB, Laws ER. Skull base chordomas: Overview of disease, management options, and outcome. Neurosurg Focus. 2001. 10: E12
17. McMaster ML, Goldstein AM, Bromley CM, Ishibe N, Parry DM. Chordoma: Incidence and survival patterns in the United States 1973-1995. Cancer Causes Control. 2001. 12: 1-11
18. Noël G, Feuvret L, Dhermain F, Mammar H, Haie-Méder C, Ponvert D. Chordomas of the base of the skull and upper cervical spine 100 patients irradiated by a 3D conformal technique combining photon and proton beams. Cancer Radiother. 2005. 9: 161-74
19. Palsetia DR, Vijan AV, Gala FB, Sahu AC, Patkar DP, Sahu AA. Clival and paraclival lesions: A pictorial review. Indian J Radiol Imaging. 2023. 33: 201-17
20. Saito K, Toda M, Tomita T, Ogawa K, Yoshida K. Surgical results of an endoscopic endonasal approach for clival chordomas. Acta Neurochir (Wien). 2012. 154: 879-86
21. Samii A, Gerganov VM, Herold C, Hayashi N, Naka T, Mirzayan MJ. Chordomas of the skull base: Surgical management and outcome. J Neurosurg. 2007. 107: 319-24
22. Sebro R, DeLaney T, Hornicek F, Schwab J, Choy E, Nielsen GP. Differences in sex distribution, anatomic location and MR imaging appearance of pediatric compared to adult chordomas. BMC Med Imaging. 2016. 16: 53
23. Seker A, Inoue K, Osawa S, Akakin A, Kilic T, Rhoton AL. Comparison of endoscopic transnasal and transoral approaches to the craniovertebral junction. World Neurosurg. 2010. 74: 583-602
24. Sekhar LN, Pranatartiharan R, Chanda A, Wright DC. Chordomas andchondrosarcomas of the skull base: Results and complications of surgical man-agement. Neurosurg Focus. 2001. 10: E2
25. Shidoh S, Toda M, Nakajima H, Tomita T, Ogawa K, Kawase T. Transoral-Transpalatal approach and endoscopic endonasal approach for chordomas of the craniovertebral junction. J Neurol Neurophysiol. 2013. 4: 4
26. Shidoh S, Toda M, Kawase T, Nakajima H, Tomita T, Ogawa K. Transoral vs. endoscopic endonasal approach for clival/upper cervical chordoma. Neurol Med Chir (Tokyo). 2014. 54: 991-8
27. Stippler M, Gardner PA, Snyderman CH, Carrau RL, Prevedello DM, Kassam AB. Endoscopic endonasal approach for clival chordomas. Neurosurgery. 2009. 64: 268-77
28. Walcott BP, Nahed BV, Mohyeldin A, Coumans JV, Kahle KT, Ferreira MJ. Chordoma: Current concepts, management, and future directions. Lancet Oncol. 2012. 13: e69-76
29. Youssef AS, Guiot B, Black K, Sloan AE. Modifications of the transoral approach to the craniovertebral junction: Anatomic study and clinical correlations. Neurosurgery. 2008. 62: 145-54
30. Zhai Y, Bai J, Wang S, Du J, Wang J, Li C. Differences in Dural penetration of clival chordomas are associated with different prognosis and expression of platelet-derived growth factor receptor-b. World Neurosurg. 2017. 98: 288-95
31. Zhou Y, Hu B, Wu Z, Cheng H, Dai M, Zhang B. The clinical outcomes for chordomas in the cranial base and spine: A single center experience. Medicine (Baltimore). 2019. 98: e15980