- Department of Brain Sciences, Imperial College of London, London, United Kingdom
- Barts and the London School of Medicine and Dentistry, Queen Mary University of London, London, United Kingdom
- Department of Oral and Maxillo-Facial Surgery, Northwick Park Hospital, London, United Kingdom
Correspondence Address:
Giulio Anichini, Department of Brain Sciences, Imperial College of London, London, United Kingdom.
DOI:10.25259/SNI_529_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Lucas Miguel Hernandez1, Nathan Chisvo2, Abigail Chan3, Kevin O’Neill1, Giulio Anichini1. Neuroepithelial cyst causing homonymous hemianopia treated through surgical marsupialization under visual-evoked potentials: A case report. 15-Nov-2024;15:419
How to cite this URL: Lucas Miguel Hernandez1, Nathan Chisvo2, Abigail Chan3, Kevin O’Neill1, Giulio Anichini1. Neuroepithelial cyst causing homonymous hemianopia treated through surgical marsupialization under visual-evoked potentials: A case report. 15-Nov-2024;15:419. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13226
Abstract
Background: Neuroepithelial cysts (NECs) are rare entities, occasionally causing neurological symptoms that can be overlooked.
Case Description: A case of an occipital neuroepithelial cyst is discussed. The initial presentation consisted of mild homonymous hemianopia and gait impairment. Conservative management was suggested to start with, but at 6 months follow-up, the patient’s symptoms were worsening. Surgery was performed under general anesthetic and using visual-evoked potentials. The cyst was marsupialized and connected with subdural space, and a few samples were sent for histological analysis. The patient experienced immediate improvement in her symptoms, and the visual tests at follow-up confirmed the resolution of the previously documented hemianopia.
Conclusion: NECs should be carefully assessed to rule out symptoms associated with mass effects. This case and others reported in the international literature show that occipital neuroepithelial cysts can benefit from surgical treatment with meticulous preoperative planning. The aid of neuromonitoring is crucial to identify anatomical variations and cortical functionality that are potentially distorted in the presence of these lesions.
Keywords: Glioependymal cyst, Neuroependymal cyst, Neuroepithelial cyst, Neuroglial cyst
INTRODUCTION
Neuroepithelial cysts (NECs), also known as neuroglial cysts (NGC), glioependymal cysts (GEC), or neuroependymal cysts, are benign developmental anomalies of the central nervous system (CNS) derived from ectodermal remnants. Their distinction from choroid plexus cysts (CPCs) is unclear, but the latest classifications tend to group these lesions.[
CASE REPORT
A 60-year-old right-handed lady was referred to our neurosurgery service by her general practitioner due to 3 months clinical history of headaches and suspected apraxia. The headache was reported as non-positional, intermittent, with a frequency of a few days, primarily retro-orbital, and not exacerbated by cough or sneezing. The patient also reported an increasingly noticeable worsening of her gait, with a tendency to trip over and deviate toward the right-side during walking.
Three different neurosurgical consultants and one neurologist saw the patient. One physician reported some possible signs related to the pathology, although there was a disagreement regarding the findings. A possible extremely subtle left-sided homonymous hemianopia located at the very extreme end of the visual field and an equivocal, minor degree of gait deviation toward the left was reported. Apart from these two possible findings, neurology was unremarkable. The patient was otherwise fit and well, working as a psychotherapist, exercising regularly, and having no significant comorbidities.
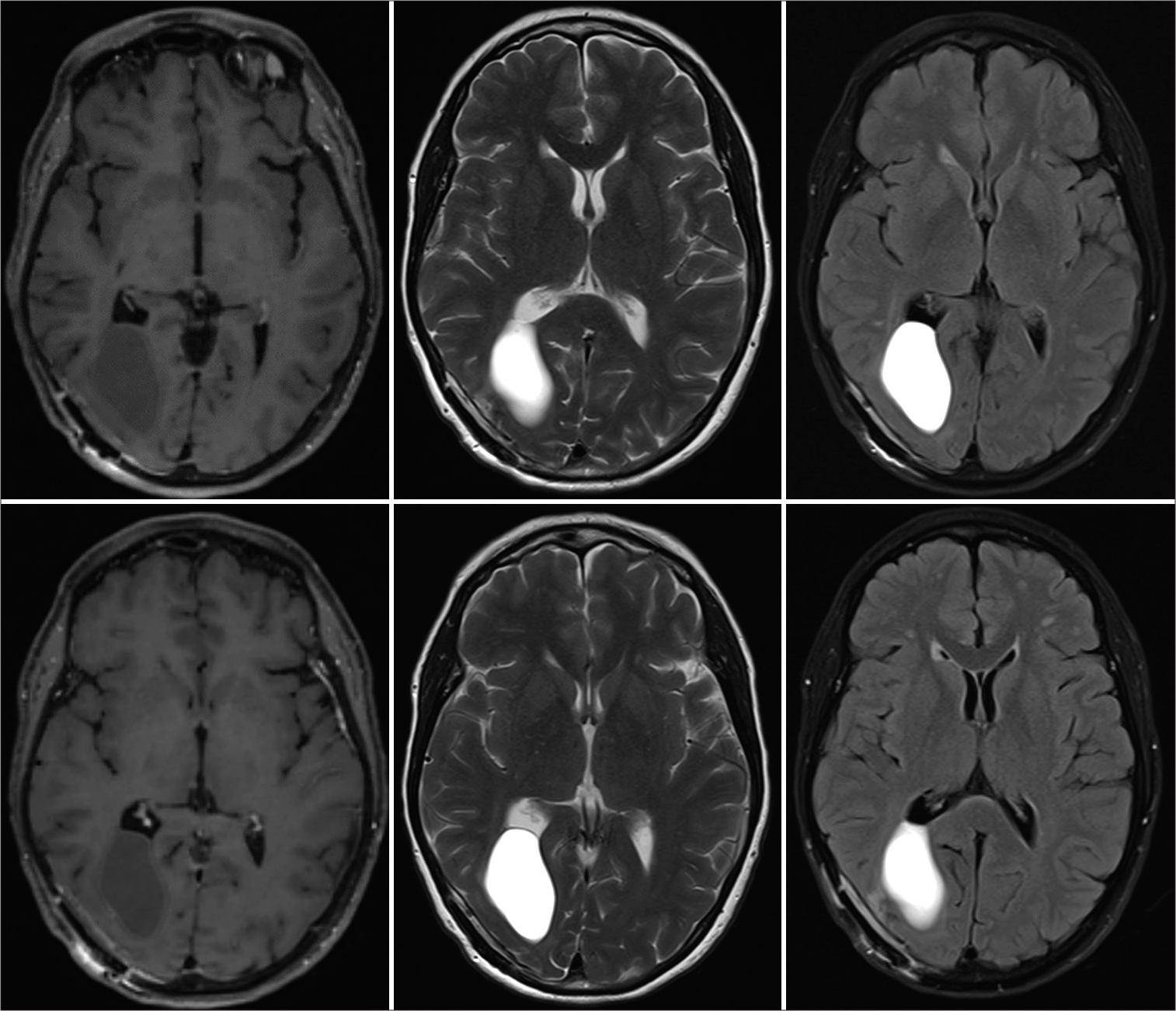
Magnetic resonance imaging (MRI) was performed to rule out an intracranial mass. The scan highlighted a right parieto-occipital multiloculated cyst. A first, more minor component was isointense to the cerebrospinal fluid (CSF) on fluid-attenuated inversion recovery sequences located in the inferoposterior portion of the cyst. A second, more prominent component showed suspected proteinaceous content and was located superior-anteriorly, in close contact with the atrium of the lateral ventricle on the same side. The cyst wall did not take contrast, and there was no restriction on diffusion sequences.
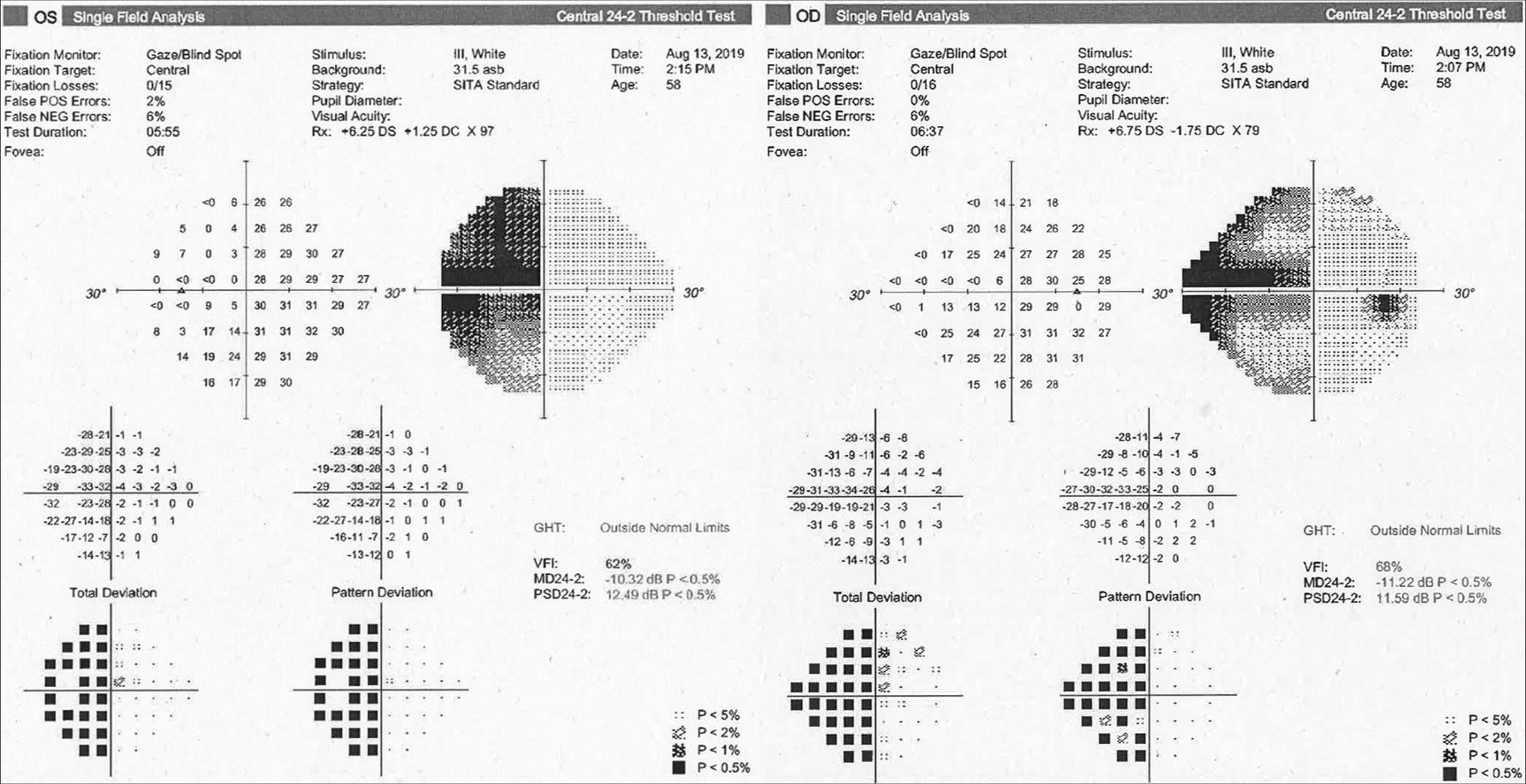
The case was discussed in the department multi-disciplinary team meeting (MDT), taking into account the high performance status of the patient and the concerns related to a potentially invasive procedure close to the visual cortex. Both surgical and conservative options were discussed with the patient. All factors considered, conservative management through a wait-and-watch strategy was initially recommended and agreed on by the patient. A formal ophthalmology assessment and an MRI scan were booked to be performed after 6 months after the initial visit. The patient was provided with active contacts during working hours and instructed to attend the hospital in an emergency setting in case of acute deterioration. At that point, on visual field assessment, the suspected homonymous hemianopia was confirmed to be present, worse on the left eye compared to the right and more marked than expected [
Surgical management and postoperative course
The surgical strategy was discussed in the MDT, and several options were considered. The aim of the surgery would have been to decompress the cyst and get a biopsy to exclude any malignancy or potentially growing lesion. Endoscopic fenestration was considered an option, but the chance of an inconclusive biopsy was considered too high. Moreover, given the expected variation in functional anatomy due to a suspected long-standing lesion, endoscopic manipulation near the visual cortex without adequately exposing the area was deemed to put the patient at risk of further visual loss. Open surgery was therefore considered, although the question of whether to put the cyst in communication with the ventricle remained. The available literature evidence was reviewed (see Discussion section): The concern of spreading proteinaceous content inside the ventricle and the literature trend that most of these procedures report successful open fenestration with no rate of recurrence,[
Surgery has been performed under total intravenous anesthesia under neurophysiological monitoring. Specifically, somatosensory-evoked potentials and visual-evoked potentials (VEPs) were running during the procedure. The patient has been positioned prone on a Montreal mattress, with the head fixed on a Mayfield clamp, and neuronavigation has been set up to identify the entry area. After disinfection and draping, a parasagittal approach and a right-sided parieto-occipital craniotomy have been performed to access the lesion.
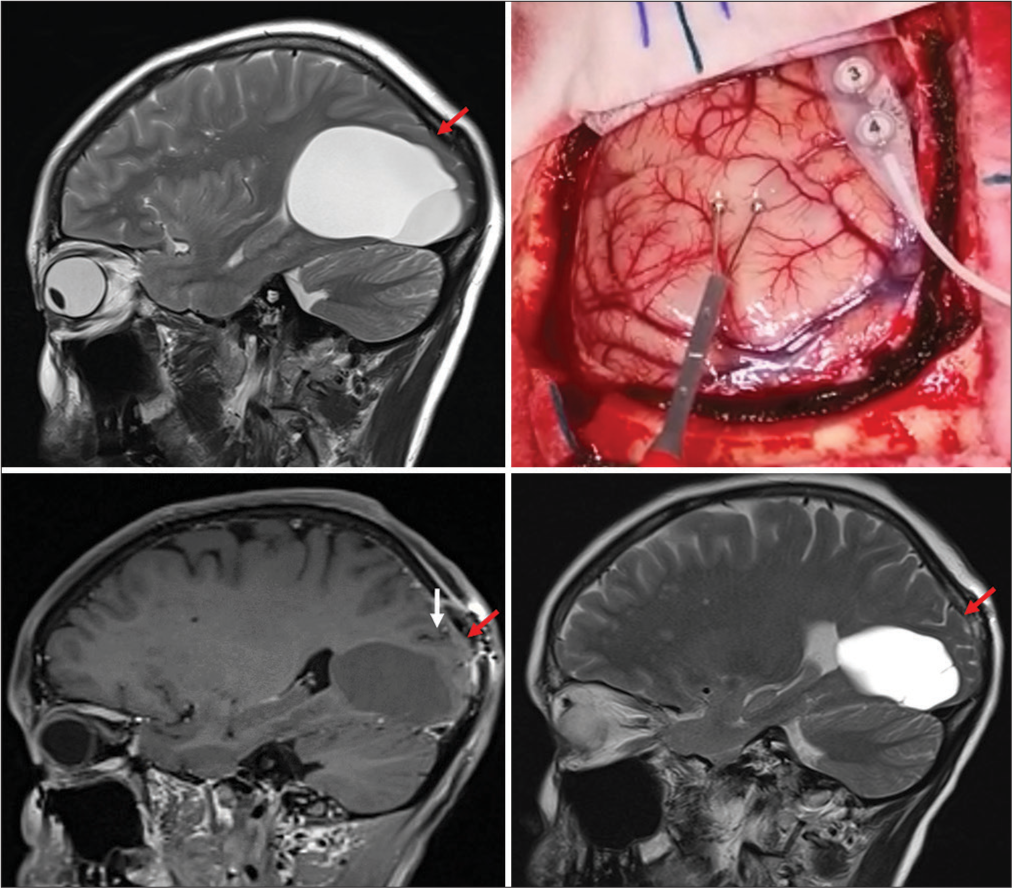
As soon as the bone flap was removed, the VEPs changed in amplitude and shape. The dura has been, therefore, opened in a curvilinear fashion with the pedicle flap towards the midline, and the cyst position has been checked using intra-operative neuronavigation. At this point, cortical stimulation has been performed to identify the calcarine cortex and the corresponding primary visual area. Interestingly, the most superficial area of the cyst was located under a highly responsive area, where clear interruption of the VEPs was achieved with a minimal amount of cortical stimulation (<3 mA). After completing the cortical mapping, a safe entry point has been established at the level of the parieto-occipital sulcus, just beneath the pre-cuneus, above a bridging vein [
Figure 3:
(Top left) Preoperative magnetic resonance imaging scan (sagittal) showing the marked compression of the occipital cortex. This was initially interpreted as non-functional/atrophic. Red arrow points towards the re-expansion of the brain tissue before and after fenestration. However, on intraoperative stimulation (top right), the most superficial point of the cortex overlying the cyst appeared to be functionally active; therefore, a more superior entry point was chosen above the draining vein seen in the surgical field. (Bottom left and bottom right) Red arrows show the re-expansion of the thin occipital cortical mantle, and white arrows point out the sulcus chosen as an entry point.
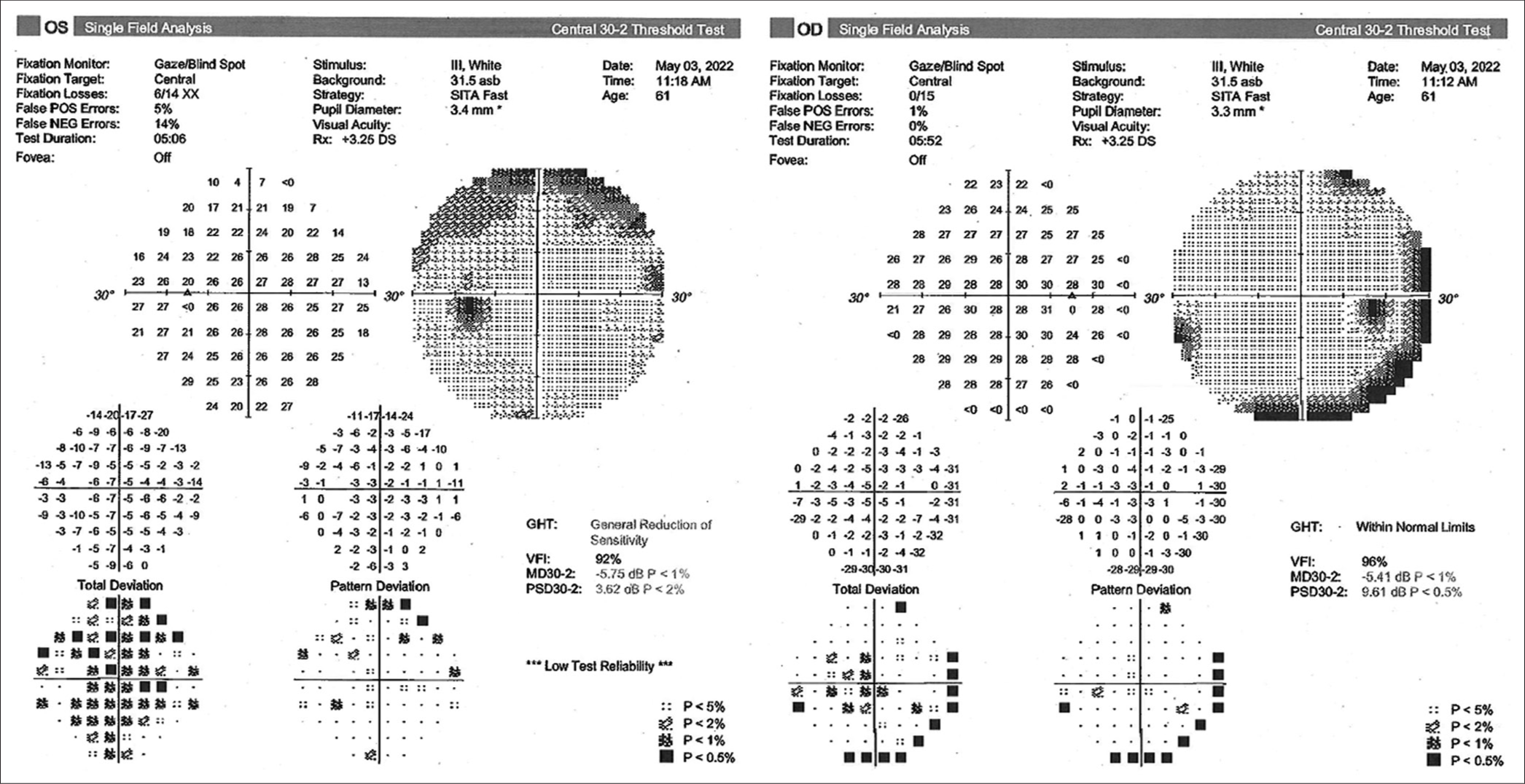
On awakening, the patient was neurologically intact, with no new neurological deficits on four limbs, cranial nerves, or changes in higher cognitive performances. She reported immediate resolution of the preoperative headache. On mobilization the next day, she did not show any obvious gait impairment, her postoperative course was uneventful, and she was discharged on day 2 postoperative. The postoperative MRI scan revealed a significant reduction in the size of the cyst on both its components [
The histology revealed a neuroepithelial cyst with cuboidal epithelium, positive for cytokeratin, epithelial membrane antigen (EMA), glial fibrillary acid protein (GFAP), and negative for S-100.
DISCUSSION
NEC is rare but often misinterpreted as congenital lesions of the CNS. According to the latest classifications, the larger NEC group includes ependymal cysts and CPCs.[
To the best of our knowledge, four reports for six cases of symptomatic occipital NEC in adults are reported in the recent medical literature.[
Interestingly, VEPs appeared to change as soon as the dura was opened. While this finding might be interpreted as a reliable adjustment of the cortex to the relieved local pressure from the cyst, VEPs are notoriously volatile and show a relatively high percentage of false positives. Therefore, caution is advised by several authors regarding their interpretation.[
CONCLUSION
Occipital neuroepithelial cysts are rare lesions that can occasionally present with symptoms and signs indicative of localized neurological compromise. Clinical manifestations may be subtle, necessitating comprehensive evaluation prior to discard any aggressive approach. Surgical intervention, when indicated, must be meticulously planned due to the cyst’s proximity to critical structures, particularly the visual cortex and optic radiations. Despite limited data, current literature evidence suggests a favorable prognosis with a high recovery rate following surgical fenestration.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
This case report did not receive fundings. However, Mr. Giulio Anichini was financially supported by Brain Tumor Research (BTR), George Pickard’s Research Fellowship, and Brain Tumor Research Campaign (BTRC).
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
Acknowledgments
Mr. Giulio Anichini was financially supported by Brain Tumor Research (BTR), George Pickard’s Research Fellowship, and Brain Tumor Research Campaign (BTRC).
References
1. Abdulateef AA, Morita S, Hoz SS, Atallah O, Numazawa S, Ito Y. Glioependymal cyst in the medulla oblongata-A case report. Surg Neurol Int. 2023. 14: 432
2. Alvarado AM, Smith KA, Chamoun RB. Neuroendoscopic fenestration of glioependymal cysts to the ventricle: Report of 3 cases. J Neurosurg. 2018. 131: 1615-9
3. Bakshi AM, Agrawal A, Bakshi SS, Kumbhare A, Chakole S. An unusual presentation of glioependymal cyst encroaching neuronal parenchyma in an elderly female: A case report. Cureus. 2023. 15: e37835
4. Basilotta Marquez Y, Gromadzyn G, Tcherbbis Testa V, Rugilo C, Argañaraz R, Mantese B. Choroid plexus cyst causing acute hydrocephalus and transtentorial herniation: Report of a rare case and its successful neuroendoscopic treatment. Childs Nerv Syst. 2022. 38: 435-9
5. Boockvar JA, Shafa R, Forman MS, O’Rourke DM. Symptomatic lateral ventricular ependymal cysts: Criteria for distinguishing these rare cysts from other symptomatic cysts of the ventricles: Case report. Neurosurgery. 2000. 46: 1229-33
6. Chaves JP, Gallo BH, E Silva NL, da Silva LL, Mattozo CA. Intracranial ependymal cyst-A modern systematic review with a pathway to diagnosis. J Clin Neurosci. 2022. 99: 10-6
7. El-Ghandour NM. Endoscopic treatment of intraventricular ependymal cysts in children: Personal experience and review of literature. Childs Nerv Syst. 2018. 34: 2441-8
8. Gangemi M, Maiuri F, Donati PA, Signorelli F, Basile D. Endoscopic surgery for monoventricular hydrocephalus. Surg Neurol. 1999. 52: 246-51
9. Guermazi A, Miaux Y, Majoulet JF, Lafitte F, Chiras J. Imaging findings of central nervous system neuroepithelial cysts. Eur Radiol. 1998. 8: 618-23
10. Gutzwiller EM, Cabrilo I, Radovanovic I, Schaller K, Boëx C. Intraoperative monitoring with visual evoked potentials for brain surgeries. J Neurosurg. 2018. 1: 654-60
11. Hamzah A, Ashqar AA, Alshanqiti M, Lary A, Samkari A. Awake craniotomy for fenestration of motor cortex neuroglial cyst: A case report. Surg Neurol Int. 2023. 14: 323
12. Hassan J, Sepulveda W, Teixeira J, Cox PM. Glioependymal and arachnoid cysts: Unusual causes of early ventriculomegaly in utero. Prenat Diagn. 1996. 16: 729-33
13. Irie K, Shimogawa T, Mukae N, Kuga D, Iwaki T, Mizoguchi M. Combined neuroendoscopic cyst wall fenestration and cyst-peritoneal shunt in an infant with glioependymal cyst. Surg Neurol Int. 2022. 13: 102
14. Kadri H, Dughly M, Agha MS, Hamed G, Abouharb R, Mackieh R. Giant supra and retrosellar glioependymal cyst presenting with only precocious puberty. Clinical study and review of the literature. Int J Surg Case Rep. 2024. 116: 109360
15. Manan HA, Franz EA, Yahya N. Functional connectivity changes in patients with brain tumours-A systematic review on resting state-fMRI. Neurol Psychiatry Brain Res. 2020. 36: 73-82
16. Mühler MR, Hartmann C, Werner W, Meyer O, Bollmann R, Klingebiel R. Fetal MRI demonstrates glioependymal cyst in a case of sonographic unilateral ventriculomegaly. Pediatr Radiol. 2007. 37: 391-5
17. Mustapha O, Allali N, Latifa C. Epilepsy as a presentation of a neuroglial cyst associated with dysgenesis of corpus callosum in a child. Case Rep Radiol. 2021. 2021: 6675071
18. Naama O, Idir A, Boulahroud O. Occipital lobe ependymal cyst with unusual presentation. J Clin Med Exp Images. 2019. 3: 9-11
19. Ozgural O, Dogan I, Solmaz S, Morali Guler T, Kahilogullari G. Transcranial endoscopic treatment of thalamic neuroepithelial cyst: Case report and review of the literature. Br J Neurosurg. 2023. 37: 659-62
20. Pařízek J, Jakubec J, Hobza V, Němečková J, Černoch Z, Šercl M. Choroid plexus cyst of the left lateral ventricle with intermittent blockage of the foramen of Monro, and initial invagination into the III ventricle in a child. Childs Nerv Syst. 1998. 14: 700-8
21. Rauf P, Aidil MN, Chan KH, Saufi A, Fadli M. Symptomatic cerebral ependymal cyst: A case report. 2019. 18: 127
22. Shah N. Prenatal diagnosis of choroid plexus cyst: What next?. J Obstet Gynaecol India. 2018. 68: 366-8
23. Ujihara M, Kobayashi M, Sasaki A, Ishizawa K, Hirata S, Wakiya K. Multiple neuroepithelial cysts of the cerebellopontine angle: Case report and review of the literature. Interdiscip Neurosurg. 2022. 29: 101572
24. Yamaguchi I, Pooh KH, Azumi M, Takagi Y. Temporal crescent syndrome caused by a lateral ventricular glioependymal cyst: Case report. J Neurosurg Pediatr. 2020. 26: 232-6