- Department of Neurosurgery, University of Oklahoma Health Sciences Center, Oklahoma, United States
Correspondence Address:
Hakeem J. Shakir, Department of Neurosurgery, University of Oklahoma Health Sciences Center, Oklahoma, United States.
DOI:10.25259/SNI_773_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Alexander R. Evans, Jack E. Stanfield, Abigail York, Shyian Jen, Hakeem J. Shakir. Emergent salvage of the vertebral artery with flow diverter pipeline stent following vessel laceration: Systematic literature review and illustrative case example. 29-Nov-2024;15:448
How to cite this URL: Alexander R. Evans, Jack E. Stanfield, Abigail York, Shyian Jen, Hakeem J. Shakir. Emergent salvage of the vertebral artery with flow diverter pipeline stent following vessel laceration: Systematic literature review and illustrative case example. 29-Nov-2024;15:448. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13250
Abstract
Background: Iatrogenic injury to neck vasculature is a potentially life-threatening complication of spine surgery. We present an illustrative case describing the use of the PipelineTM Embolization Device (PED) in the emergent reconstruction of the vertebral artery (VA) following vessel laceration. In addition, we document a systematic review concerning the use of the PED in acute to chronic iatrogenic injury of the internal carotid or VAs.
Methods: This study was a systematic literature review and illustrative case example.
Results: A 73-year-old woman underwent anterior cervical discectomy and fusion (ACDF) surgery complicated by left VA injury. The incision was promptly packed and pressure held while the vessel was salvaged using a PipelineTM stent. At 6 months follow-up, the patient had no residual symptoms. A systematic review identified 11 publications meeting study criteria, in which 16 patients were reported to have an injury to the internal carotid or VAs. Patients were grouped into acute, subacute, and chronic cohorts. In the acute group, the majority of patients experienced injury during transsphenoidal resection or ACDF procedures. All cases in the acute group received immediate intervention with the deployment of a PED device. One patient experienced continued contrast extravasation, necessitating vessel sacrifice through superficial temporal artery-middle cerebral artery bypass. All patients recovered to their neurologic baseline. In the subacute and chronic groups, two patients experienced complications, with the majority going on to recover to their neurologic baseline.
Conclusion: PED placement is a viable management strategy for restoring anatomic integrity to head-and-neck vasculature following acute iatrogenic injury.
Keywords: Flow diverter technology, Iatrogenic vascular injury, Internal carotid artery, Pipeline embolization device, Vertebral artery
INTRODUCTION
Iatrogenic injury to the internal carotid or vertebral arteries (VAs) is a rare and potentially life-threatening complication of endoscopic endonasal or spinal decompression/fusion procedures.[
The PipelineTM Embolization Device (PED, Covidien/Medtronic, Irvine, CA) belongs to a class of flow-diverting stents, which utilizes a 48-strand cobalt chromium and platinum tungsten braided mesh design to provide radial force in the redirection of blood flow away from aneurysms.[
MATERIALS AND METHODS
A systematic literature review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2020 guidelines[
Articles were then screened by title and/or abstract based on the proper inclusion and exclusion criteria. Articles included were any original-research articles in the English language discussing the use of flow diverter pipeline stenting to salvage iatrogenic injury to the ICA or VA in adult patients. Articles were excluded if they were review papers, meta-analyses, letters/editorials, abstracts published from academic conferences, studies on non-human animals, non-clinical studies, or discussed the use of pipeline flow diverter stents in non-traumatic aneurysm dissection, general aneurysm repair, or traumatic (non-iatrogenic) vessel injury. Articles were then imported and screened through Rayyan, a web-based screening tool.[
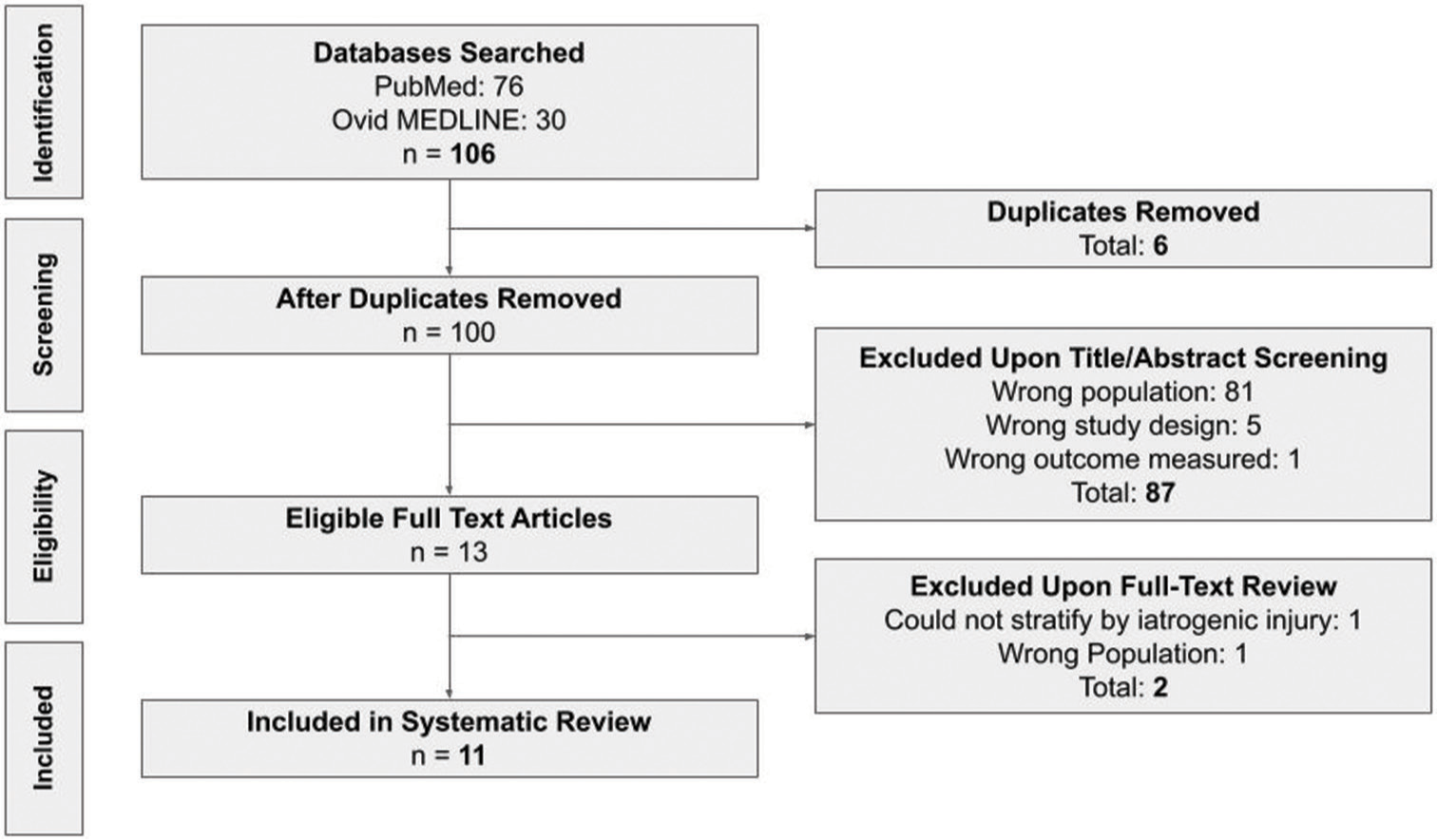
Following an initial screening, the same two reviewers carefully conducted a full-text analysis of the articles, and any conflicts were discussed between the reviewers. Data extraction had the following primary outcomes: (1) patient demographics, including the number of patients in each study and the age and biological sex of each patient; (2) description of injury, including the location of the injury, primary pathology, and surgery type; (3) symptoms at presentation, including angiographic findings and the interval between injury and treatment; and (4) outcome. Other outcome data collected included procedural complications, anticoagulation details, follow-up period, and description of stent(s). Publications were then separated into three groups: Those undergoing treatment for acute, subacute, or chronic iatrogenic vascular injury. The acute group was defined as vessel compromise necessitating immediate intervention (<24 h), whereas subacute was defined as any injury repaired within 1 month, and chronic was defined as any injury repaired beyond 1 month. A good outcome was defined as minimal or no neurological deficits at follow-up. The details of the screening process are shown in
RESULTS
Illustrative case example
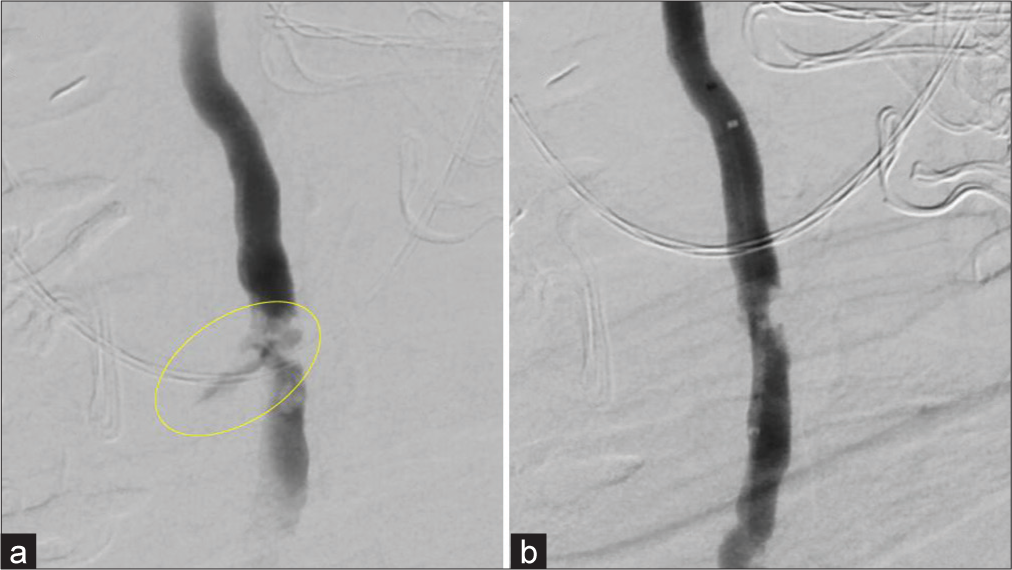
A 73-year-old woman with a medical history notable for antiphospholipid syndrome underwent anterior cervical discectomy and fusion (ACDF) surgery at the C6-C7 vertebral level at a community hospital, which was complicated by injury to and dissection of the left VA. The patient’s incision site was packed, whereupon she received two units of packed red blood cells (pRBCs) and was transferred to our institution. On arrival, a diagnostic cerebral angiogram was performed, which revealed active extravasation of contrast and confirmed injury to the proximal V2 segment of the left VA at the C6 vertebral level [
Figure 2:
Cerebral angiogram demonstrating (a) extravasation of contrast following iatrogenic injury to the proximal V2 segment of the vertebral artery and (b) successful placement of Medtronic pipeline flow diverting stent placement with salvage of the artery. Yellow circle indicates active contrast extravasation.
The decision was made to salvage the vessel using the PED, in which she received 6000 units of heparin intravenously (IV), along with an eptifibatide bolus and 90 mcg/kg drip. A benchmark guide catheter was then placed in the origin of the left VA, and the injured segment was traversed through a HeadwayTM 27 microcatheter (MicroVention, California, USA) over an Aristotle® 0.14 microwire (Scientia Vascular, Inc, Utah, USA). A 4.25 mm×16 mm PipelineTM flow diverting stent (Medtronic, California, USA) was delivered through the microcatheter and deployed across the injured segment. Contrast injection through the guiding catheter revealed no further extravasation and patency of the VA [
The patient was taken promptly to the operating room for hemostasis and closure of the ACDF incision. Intraoperatively, she required hemodynamic support with a transfusion of three additional units of pRBCs and norepinephrine. The patient then recovered well with close monitoring in the neurointensive care unit and experienced no complications. She was extubated on postoperative day one with no neurological deficits and transitioned to dual antiplatelet therapy, including oral aspirin and apixaban (81 mg and 5 mg daily by mouth, respectively). She was then discharged on postoperative day 3 to the outside location for completion of the ACDF, and no further complications were encountered. At 6 months follow-up, she was noted to have no focal deficits. Angiography at that time redemonstrated the healed VA with excellent apposition of the PED and no evidence of aneurysm [
Literature review
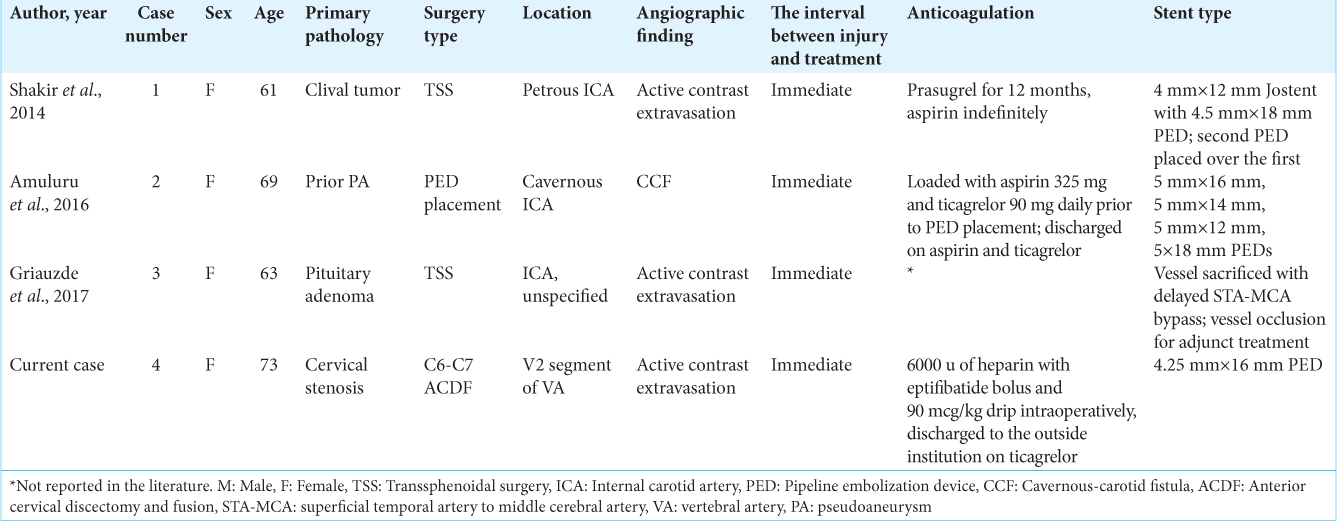
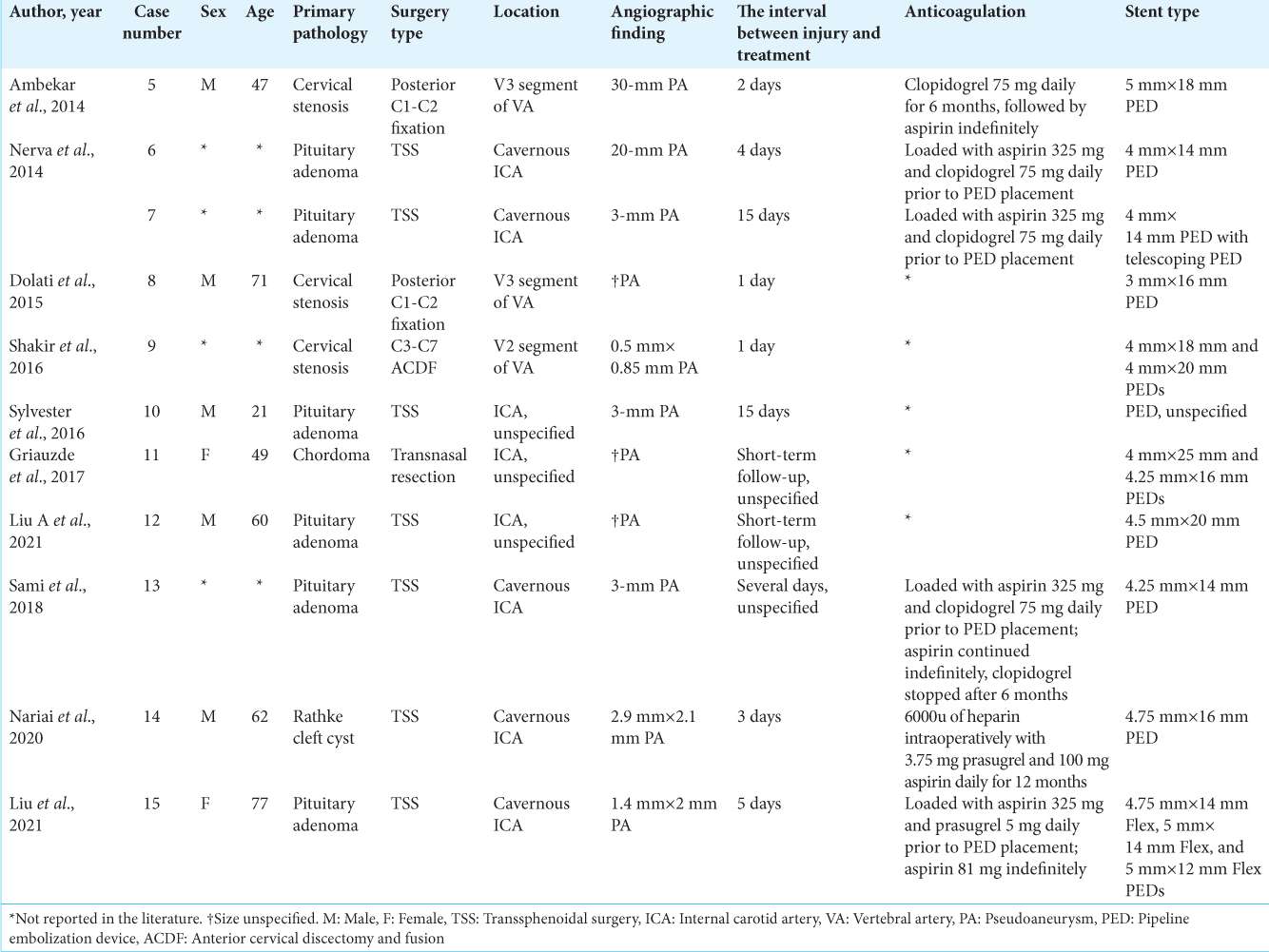
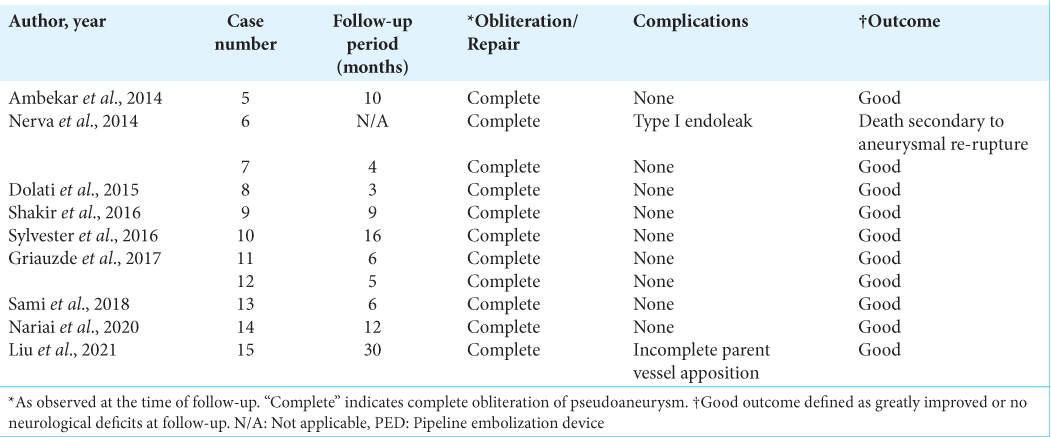
Eleven publications with sixteen patients were identified for this review [
In the acute group (n = 4), the average age for vascular injury was 66.5 (range, 61–73) years with a female predominance (ratio, 4:0). One patient experienced an injury to the petrous of the ICA while undergoing transsphenoidal surgery (TSS) for a clival tumor whereas another patient experienced an injury to an unspecified portion of the ICA while undergoing TSS for a pituitary adenoma. One patient experienced an injury to the cavernous ICA during PED placement for a prior pseudoaneurysm (PA), and the last patient experienced an injury to the V2 segment of the VA during ACDF for cervical stenosis. All patients were found to have active extravasation of contrast on angiography, barring case 2, who were found to have a cavernous-carotid fistula. All cases were deemed emergent due to the risk of hemodynamic instability. The time interval between injury and treatment was immediate in all cases. In one case, PED placement resulted in continued contrast extravasation, which necessitated vessel sacrifice through a delayed superficial temporal artery-middle cerebral artery (STA-MCA) bypass. No complications were reported, and all patients experienced good neurologic outcomes. The average follow-up time was 5.3 (range, 1–12) months.
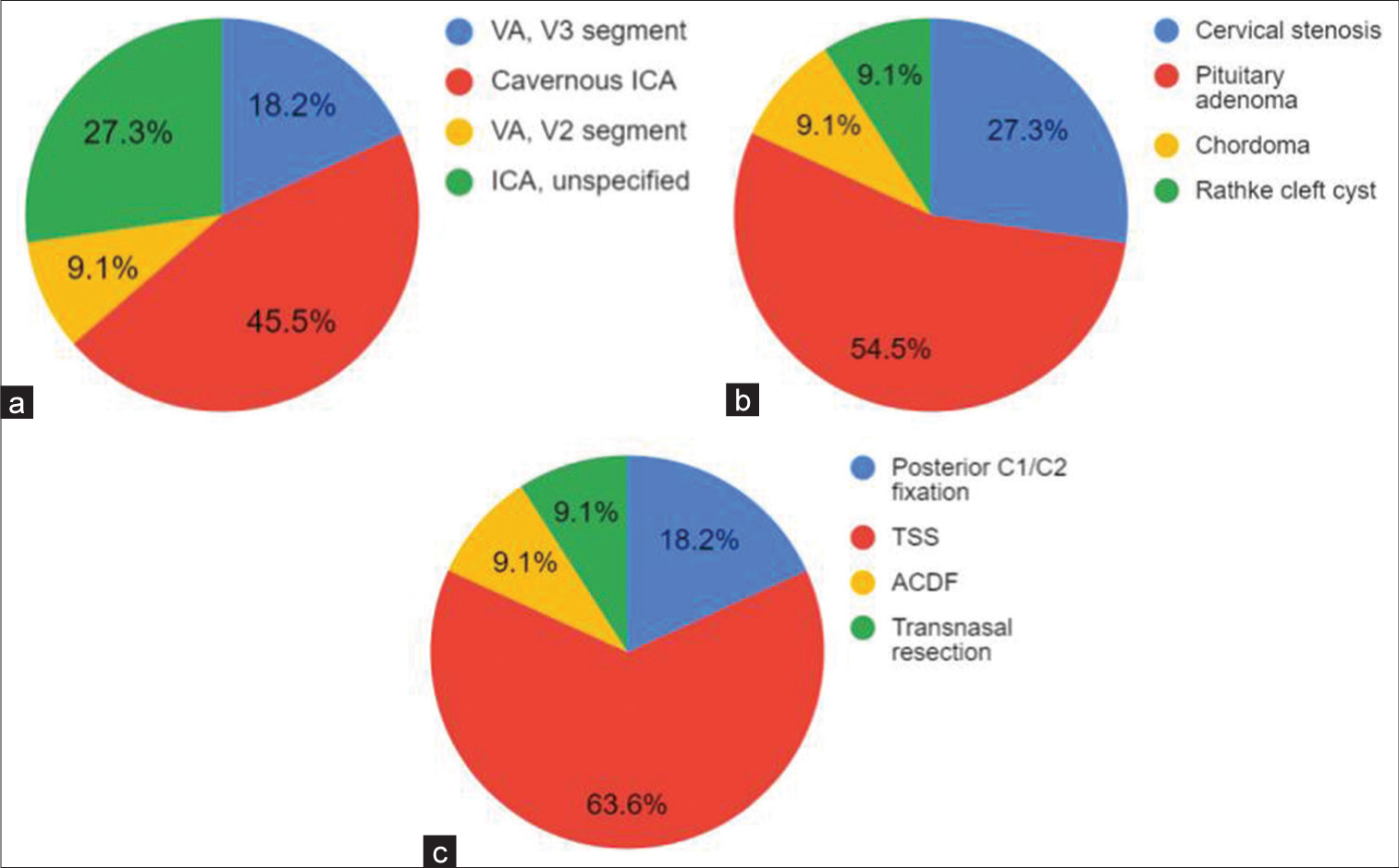
In the subacute group (n = 11), the average age at vascular injury was 55.3 (range, 21–77) years with a male predominance (ratio, 5:2). Seven patients experienced vascular injury while undergoing TSS, six being for pituitary adenoma and one being for Rathke cleft cyst. Three patients underwent decompression for cervical stenosis, with two patients experiencing vascular injury during C1/C2 fixation and one during ACDF. Five patients experienced injury to the cavernous ICA, whereas one experienced injury to segment V2 of the VA, and another experienced injury to segment V3 of the VA. Three patients experienced injury to an unspecified portion of the ICA. All patients were found to have a PA on angiography. The time interval between injury and treatment ranged from 1 to 15 days. Two patients had an immediate postoperative complication, including type I endoleak, resolved with balloon angioplasty, and incomplete parent vessel apposition, resolved with a third PED placement. All cases resulted in complete PA obliteration, with ten patients experiencing a good neurologic outcome. One death was reported. The average follow-up time was 10.1 (range, 3–30) months. Proportions of injury location, primary pathology, and procedure type in patients receiving PED placement for the treatment of subacute iatrogenic vascular injury are shown in
Figure 4:
(a) Location of vessel injury, (b) primary pathology, and (c) procedure type in patients receiving pipeline embolization device placement for the treatment of following subacute iatrogenic vascular injury. VA: Vertebral artery, ICA: Internal carotid artery, TSS: Transsphenoidal surgery, ACDF: Anterior cervical discectomy and fusion.
In the chronic group (n = 2), age was reported in one patient, who was 78 years old at the time of injury. Both patients experienced vascular injury while undergoing TSS for pituitary adenoma. One patient experienced injury to the cavernous ICA, whereas the second experienced injury to an unspecified portion of the ICA. Both patients were found to have a PA on angiography. The time interval between injury and treatment was 20 years in both cases. No complications were reported; complete or near-complete obliteration of the PA was achieved. Both patients experienced a good neurologic outcome. Follow-up time ranged from 1 day to 12 months.
DISCUSSION
We report the seventeenth iatrogenic injury of the ICA or VA treated with a PED and the fourth use of a PED in the acute setting.[
All patients in the subacute group underwent PED placement for the obliteration of a PA, in which two complications and 1 death occurred. Nonetheless, the majority of patients in this group experienced full neurologic recovery at the time of follow-up. In the chronic group, no complications were reported, and both patients recovered to their neurologic baseline following obliteration of a PA through PED placement. Altogether, PED placement has demonstrated high fidelity in salvaging the ICA or VA via reconstitution of vessel architecture. Historically, vessel sacrifice has been established as a viable strategy for restoring hemodynamic stability in the presence of adequate collateral blood flow; however, flow diversion allows for the preservation of critical vasculature in high-risk cases.
Current literature regarding the management and outcomes of iatrogenic injury to the ICA or VA remains limited. Although rare overall, the cavernous segment of the ICA appears to be the most common site of iatrogenic injury during endoscopic endonasal surgery. Conventional treatment modalities include covered stent placement or coil embolization,[
Within the context of iatrogenic VA injury, drilling and instrumentation significantly increase the risk of vessel compromise during anterior cervical spine operations.[
Flow diverter technology is promising in the management of acute iatrogenic vascular injury to the internal carotid or VAs. Distinct advantages of this technology include enhanced endothelialization that may limit the duration of dual antiplatelet therapy (through advancements in stent coatings) and the preservation of critical vasculature. However, dual-antiplatelet therapy is currently required for 6 months following treatment. Altogether, the PipelineTM stent should be heavily considered as a definitive intervention in the management of acute injuries to head and neck vessels.
Limitations
The current work is limited by several variables intrinsic to observational studies, chiefly being small sample size. In addition, the timeline between vascular injury and PED placement varied widely, as some PAs were not diagnosed until several years following the patient’s initial procedure. Considering that the ICA and VA are significantly different anatomically, the conclusions of this review are limited by the lack of homogenous data available in the literature. The cohort of 17 patients remains significantly small, in larger cohort studies are recommended to elucidate the efficacy of PED placement for acute iatrogenic injury of head and neck vasculature.
CONCLUSION
In this illustrative case and systematic review, we report the promising use of the PED in the context of ICA or VA injury and a range of acute to chronic pathology, resulting in high technical success rates with salvage of critical vasculature. In the current literature, iatrogenic injury commonly occurs to the cavernous segment of the ICA during transsphenoidal resection but may also occur to the VA in posterior cervical instrumentation and/or ACDF procedures. Further, we found that PED placement may be a vital modality for restoring anatomical integrity to the ICA or VA during acute injury, with the majority of patients experiencing optimal clinical outcomes and a complete or near-complete return to the neurologic baseline.
Ethical approval
The Institutional Review Board approval is not required as this report contained fewer than two patients, so ethical approval was not required by the University of Oklahoma Health Science Center’s Institutional Review Board.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Ambekar S, Sharma M, Smith D, Cuellar H. Successful treatment of iatrogenic vertebral pseudoaneurysm using pipeline embolization device. Case Rep Vasc Med. 2014. 2014: 341748
2. Amuluru K, Al-Mufti F, Gandhi CD, Prestigiacomo CJ, Singh IP. Direct carotid-cavernous fistula: A complication of, and treatment with, flow diversion. Interv Neuroradiol. 2016. 22: 569-76
3. Barrie U, Detchou D, Reddy R, Tao J, Elguindy M, Reimer C. Vertebral artery injury with anterior cervical spine operations: A systematic review of risk factors, clinical outcomes, and management strategies. World Neurosurg. 2023. 173: 226-36.e12
4. Dolati P, Eichberg DG, Thomas A, Ogilvy C. Application of pipeline embolization device for iatrogenic pseudoaneurysms of the extracranial vertebral artery: A case report and systematic review of the literature. Cureus. 2015. 7: e356
5. Ghorbani M, Griessenauer CJ, Shojaei H, Wipplinger C, Hejazian E. Endovascular reconstruction of iatrogenic internal carotid artery injury following endonasal surgery: A systematic review. Neurosurg Rev. 2021. 44: 1797-804
6. Griauzde J, Ravindra VM, Chaudhary N, Gemmete JJ, Mazur MD, Roark CD. Use of the Pipeline embolization device in the treatment of iatrogenic intracranial vascular injuries: A bi-institutional experience. Neurosurg Focus. 2017. 42: E9
7. Guan Q, Chen L, Long Y, Xiang Z. Iatrogenic vertebral artery injury during anterior cervical spine surgery: A systematic review. World Neurosurg. 2017. 106: 715-22
8. Liu A, Rincon-Torroella J, Bender MT, McDougall CG, Tufaro AP, London NR. Combined pipeline embolization device with endoscopic endonasal fascia lata/muscle graft repair as a salvage technique for treatment of iatrogenic carotid artery pseudoaneurysm. J Neurol Surg Rep. 2021. 82: e43-8
9. Nariai Y, Kawamura Y, Takigawa T, Hyodo A, Suzuki K. Pipeline embolization for an iatrogenic intracranial internal carotid artery pseudoaneurysm after transsphenoidal pituitary tumor surgery: Case report and review of the literature. Interv Neuroradiol. 2020. 26: 74-82
10. Nerva JD, Morton RP, Levitt MR, Osbun JW, Ferreira MJ, Ghodke BV. Pipeline embolization device as primary treatment for blister aneurysms and iatrogenic pseudoaneurysms of the internal carotid artery. J Neurointerv Surg. 2015. 7: 210-6
11. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016. 5: 210
12. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021. 372: n71
13. Sami MT, Gattozzi DA, Soliman HM, Reeves AR, Moran CJ, Camarata PJ. Use of PipelineTM embolization device for the treatment of traumatic intracranial pseudoaneurysms: Case series and review of cases from literature. Clin Neurol Neurosurg. 2018. 169: 154-60
14. Shakir H, Garson A, Sorkin G, Mokin M, Eller J, Dumont T. Combined use of covered stent and flow diversion to seal iatrogenic carotid injury with vessel preservation during transsphenoidal endoscopic resection of clival tumor. Surg Neurol Int. 2014. 5: 81
15. Shakir H, Rooney P, Rangel-Castilla L, Yashar P, Levy E. Treatment of iatrogenic V2 segment vertebral artery pseudoaneurysm using Pipeline flow-diverting stent. Surg Neurol Int. 2016. 7: 104
16. Shin DS, Carroll CP, Elghareeb M, Hoh BL, Kim BT. The evolution of flow-diverting stents for cerebral aneurysms; historical review, modern application, complications, and future direction. J Korean Neurosurg Soc. 2020. 63: 137-52
17. Sylvester PT, Moran CJ, Derdeyn CP, Cross DT, Dacey RG, Zipfel GJ. Endovascular management of internal carotid artery injuries secondary to endonasal surgery: Case series and review of the literature. J Neurosurg. 2016. 125: 1256-76