- Department of Neurosurgery Westchester Medical Center, Valhalla, New York, United States
- Department of Otolaryngology-Head and Neck Surgery, Westchester Medical Center, Valhalla, New York, United States
Correspondence Address:
Jared M. Pisapia, Department of Neurosurgery, Westchester Medical Center, Valhalla, New York, United States.
DOI:10.25259/SNI_663_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Sabrina L Zeller1, Michael G. Kim1, Fawaz Al-Mufti1, Simon J. Hanft1, Matthew Kim2, Jared M. Pisapia1. Safety of emergency endoscopic endonasal surgery in COVID-positive patients with hemorrhagic complications of pituitary region tumors: A case report and review of the literature. 13-Dec-2024;15:460
How to cite this URL: Sabrina L Zeller1, Michael G. Kim1, Fawaz Al-Mufti1, Simon J. Hanft1, Matthew Kim2, Jared M. Pisapia1. Safety of emergency endoscopic endonasal surgery in COVID-positive patients with hemorrhagic complications of pituitary region tumors: A case report and review of the literature. 13-Dec-2024;15:460. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13289
Abstract
Background: Pituitary apoplexy (PA) is a rare, life-threatening clinical syndrome that occurs in response to acute ischemic infarction or hemorrhage of a pituitary adenoma. We report two cases of sudden neurologic and visual decline in patients with pituitary region masses in coronavirus disease (COVID)-positive patients with a focus on potential pathophysiological mechanisms and a safe approach to treatment.
Case Description: Case one is a 58-year-old male presenting with sudden-onset headache and visual disturbance. He was febrile and tested positive for COVID-19. Magnetic resonance imaging (MRI) revealed a large sellarsuprasellar mass with intratumoral hemorrhagic components. He underwent endoscopic endonasal resection with subsequent improvement in vision and oculomotor function. Pathology was consistent with hemorrhagic pituitary adenoma. Case two is a 15-year-old male presenting with sudden-onset severe headache and acute visual loss. He also tested positive for COVID-19. MRI revealed a sellar-suprasellar mass with a regional mass effect. He underwent endoscopic endonasal resection with improvement in vision over time. Pathology was consistent with craniopharyngioma. There was no evidence of intraoperative COVID-19 transmission among members of the surgical team, who were monitored for 2 weeks after surgery.
Conclusion: PA in the setting of severe acute respiratory syndrome coronavirus 2 infection should be considered in the differential diagnosis of a COVID-positive patient presenting with acute severe headache, visual loss, and/or ophthalmoplegia; we discuss proposed mechanisms related to inflammation, coagulability, and hypoxia. The absence of intraoperative COVID-19 transmission during transsphenoidal resection performed in an emergency setting suggests that the risk of exposure may be attenuated with safety precautions.
Keywords: Apoplexy, Coronavirus disease 2019, Pituitary, Severe acute respiratory syndrome coronavirus 2, Transsphenoidal
INTRODUCTION
Pituitary apoplexy (PA) is a rare and life-threatening clinical syndrome that occurs in response to acute ischemic infarction or hemorrhage of a pituitary lesion with rapid expansion of sellar contents.[
Coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is largely recognized as a severe respiratory viral syndrome. However, COVID-19 is also known to be associated with systemic alterations, such as pro-inflammatory and hypercoagulable states, that likely contribute to neurological complications, such as stroke.[
CASE DESCRIPTION
Case 1
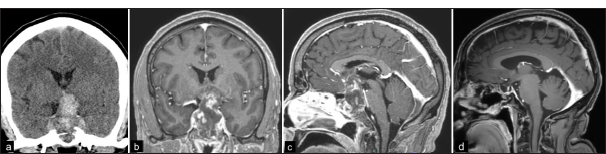
A 58-year-old male with no past medical history presented with sudden onset of headache, nausea, vomiting, and decreased visual acuity on the day of admission. He had no upper respiratory symptoms. He had not received vaccination against COVID-19 and was not receiving any COVID-19 treatments. He was febrile to 101.3°F with otherwise stable vital signs. On examination, he was confused and intermittently followed commands. Examination of the right eye revealed complete ophthalmoplegia with a visual acuity of 20/400. The left eye had reduced visual acuity and was only able to finger count; however, extraocular movement was intact. On confrontation, the patient was noted to have bitemporal hemianopsia. There were no other neurologic deficits noted on examination. A computed tomography (CT) scan of the head showed a sellar/suprasellar mixed-density mass with osseous remodeling of the sella turcica and hyperdensity within portions of the mass consistent with blood products [
Figure 1:
(a) Non-contrast head CT shows a predominantly hyperdense mass in the sellar/suprasellar region representing intratumoral density and/or blood products. (b, c) Coronal and sagittal T1-weighted MRI with contrast show a 5 cm heterogeneous mass with intratumoral blood products and significant compression of the optic apparatus. *(d) Postoperative MRI shows no evidence of residual or recurrence.
Case 2
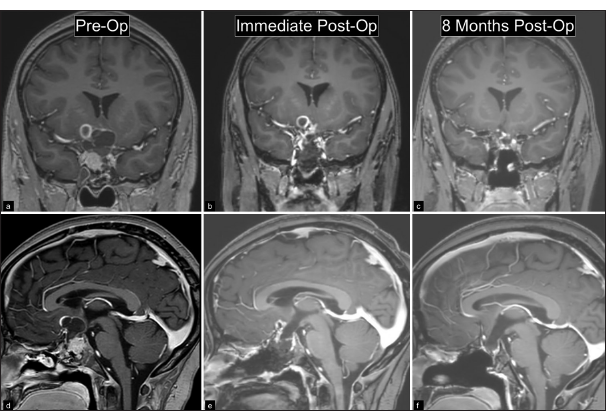
A 15-year-old male with a history of hypothyroidism and adrenal insufficiency status post transsphenoidal resection of a reported pituitary macroadenoma in another country 5 years prior presented with a sudden decline in visual acuity. Per the report, the patient had a residual tumor that was followed with surveillance imaging and had not received any adjuvant therapy. Baseline visual deficits following his prior surgery included the ability only to see shadows in the left eye and a 40% visual field cut in the right eye. In the current presentation, the patient reported a progressive decline in visual acuity over 3 days, followed by a sudden onset of severe headache, which awoke him from sleep and complete loss of vision. He also noted new polyuria and polydipsia. He had no upper respiratory symptoms and was afebrile. He had not received a vaccination against COVID-19 and was not receiving any COVID-19 treatments. On examination, the patient had complete bilateral loss of vision without evidence of papilledema; he was otherwise neurologically intact. Head CT demonstrated a partially hemorrhagic suprasellar mass with cystic and solid components without calcifications. MRI showed a 3.8 × 2.6 × 2.3 cm heterogenous sellar/suprasellar mass with marked regional mass effect and compression of the optic apparatus [
Figure 2:
(a, b) Preoperative coronal and sagittal T1-weighted MRI with contrast show a 3.8 cm predominantly solid sellar and predominantly cystic suprasellar mass. (c, d) Immediate postoperative MRI shows resection of the mass with small cystic residual with peripheral enhancement in the right suprasellar region. (e, f) MRI with contrast at most recent follow-up following radiation therapy shows no recurrence and decreased size of residual tumor.
DISCUSSION
We report two cases of pituitary region tumors in COVID-positive patients who underwent an emergency transsphenoidal approach for resection. The purpose of this report is to raise awareness of a potential association between COVID-19 and PA or apoplexy-like events in other pituitary region tumors and to demonstrate that the two conditions may be managed safely through an endoscopic endonasal transsphenoidal approach with respect to both the patient and the treating medical team.
Understanding of the pathogenesis of PA is evolving and multifactorial. Several mechanisms relate to reduced blood supply and subsequent infarction with a susceptibility to hemorrhage. Rapid tumor growth may outstrip normal blood supply and lead to compression of the superior hypophyseal artery against the diaphragmatic notch, further limiting blood supply.[
Multiple features of the COVID-19 virus provide plausible mechanisms that may contribute to a role in apoplexy or acute hemorrhage of a parasellar mass. COVID-19 virus accesses host cells using an angiotensin-converting enzyme 2 receptor, which is found in neurons and glial cells.[
Additional support for a relationship between COVID and PA relates to the interplay between hypoxia and ischemia. Patients with severe COVID-19 often develop pneumonia and acute respiratory distress syndrome, resulting in diffuse alveolar damage and hypoxemia.[
The occurrence of acute hemorrhagic or hemorrhagic infarct of a pituitary region mass in the setting of a COVID-positive diagnosis can make management more challenging. Procedures that involve the nasal mucosa, where SARS-CoV-2 is known to colonize, raise safety concerns related to airway management and disease transmission. Zhu et al. reported a case performed early during the COVID-19 pandemic where a COVID-19 patient underwent a transsphenoidal pituitary adenoma resection that led to 14 cases of postoperative COVID-19 transmission among the medical staff.[
We report the safe use of the transsphenoidal technique in two COVID-positive patients. The transsphenoidal approach was used because it was felt to be associated with improved visualization and decreased operative time when compared to an open cranial approach. An endonasal approach using a speculum and microscope was considered; however, the use of surgical loupes or an operating microscope for an extended period while wearing a face shield and N95 would have likely been cumbersome to the surgeon. Due to the known aerosolizing capabilities of microdebriders, electrocautery, and especially high-speed drills, attempts were made to minimize any unneeded debridement of the nasal mucosa and to rarely use any powered instrumentation to minimize the risk of viral exposure.[
CONCLUSION
For a COVID-positive patient presenting with acute severe headache, vision loss, and/or ophthalmoplegia, acute hemorrhage or hemorrhagic infarction of a sellar or suprasellar mass should be considered. The association between SARS-CoV-2 infection and hemorrhagic complications of pituitary region tumors may relate to mechanisms such as inflammation, coagulability, and hypoxia. Transsphenoidal surgery in the sellar or suprasellar region performed in an emergency setting in a COVID-positive patient was not associated with virus transmission when the appropriate safety precautions were taken.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Aghayari Sheikh Neshin S, Shahjouei S, Koza E, Friedenberg I, Khodadadi F, Sabra M. Stroke in SARS-CoV-2 infection: A pictorial overview of the pathoetiology. Front Cardiovasc Med. 2021. 8: 649922
2. Akhter N, Ahmad S, Alzahrani FA, Dar SA, Wahid M, Haque S. Impact of COVID-19 on the cerebrovascular system and the prevention of RBC lysis. Eur Rev Med Pharmacol Sci. 2020. 24: 10267-78
3. Baig AM, Khaleeq A, Ali U, Syeda H. Evidence of the COVID-19 virus targeting the CNS: Tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020. 11: 995-8
4. Barkhoudarian G, Kelly DF. Pituitary apoplexy. Neurosurg Clin N Am. 2019. 30: 457-63
5. Bhoelan S, Langerak T, Noack D, van Schinkel L, van Nood E, van Gorp EC. Hypopituitarism after orthohantavirus infection: What is currently known?. Viruses. 2019. 11: 340
6. Biousse V, Newman NJ, Oyesiku NM. Precipitating factors in pituitary apoplexy. J Neurol Neurosurg Psychiatry. 2001. 71: 542-5
7. Bordes SJ, Phang-Lyn S, Najera E, Borghei-Razavi H, Adada B. Pituitary apoplexy attributed to COVID-19 infection in the absence of an underlying macroadenoma or other identifiable cause. Cureus. 2021. 13: e13315
8. Briet C, Salenave S, Bonneville JF, Laws ER, Chanson P. Pituitary apoplexy. Endocr Rev. 2015. 36: 622-45
9. Carsana L, Sonzogni A, Nasr A, Rossi RS, Pellegrinelli A, Zerbi P. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: A two-centre descriptive study. Lancet Infect Dis. 2020. 20: 1135-40
10. Chan JL, Gregory KD, Smithson SS, Naqvi M, Mamelak AN. Pituitary apoplexy associated with acute COVID-19 infection and pregnancy. Pituitary. 2020. 23: 716-20
11. Chen Y, Hu F, Wang J, Huang K, Liu W, Tan Y. Clinical features of craniopharyngioma with tumoral hemorrhage: A retrospective case-controlled study. Front Surg. 2022. 9: 845273
12. D’Ignazio L, Bandarra D, Rocha S. NF-κB and HIF crosstalk in immune responses. FEBS J. 2016. 283: 413-24
13. Fernandez A, Karavitaki N, Wass JA. Prevalence of pituitary adenomas: A community-based, cross-sectional study in Banbury (Oxfordshire, UK). Clin Endocrinol (Oxf). 2010. 72: 377-82
14. Fleseriu M, Buchfelder M, Cetas JS, Fazeli PK, Mallea-Gil SM, Gurnell M. Pituitary society guidance: Pituitary disease management and patient care recommendations during the COVID-19 pandemic-an international perspective. Pituitary. 2020. 23: 327-37
15. Frara S, Allora A, Castellino L, di Filippo L, Loli P, Giustina A. COVID-19 and the pituitary. Pituitary. 2021. 24: 465-81
16. Ghosh R, Roy D, Roy D, Mandal A, Dutta A, Naga D. A rare case of SARS-CoV-2 infection associated with pituitary apoplexy without comorbidities. J Endocr Soc. 2021. 5: bvaa203
17. Kellen RI, Burde RM, Hodges FJ. Occult pituitary apoplexy associated with craniopharyngioma. J Neuroophthalmol. 1988. 8: 99-104
18. Kim MG, Stein AA, Overby P, Kleinman G, Nuoman R, Gulko E. Fatal cerebral edema in a child with COVID-19. Pediatr Neurol. 2021. 114: 77-8
19. Kim YH, Cho YH, Hong SH, Kim JH, Kim MS, Khang SK. Postoperative neurologic outcome in patients with pituitary apoplexy after transsphenoidal surgery. World Neurosurg. 2018. 111: e18-23
20. LaRovere KL, Riggs BJ, Poussaint TY, Young CC, Newhams MM, Maamari M. Neurologic involvement in children and adolescents hospitalized in the United States for COVID-19 or multisystem inflammatory syndrome. JAMA Neurol. 2021. 78: 536-47
21. LaRoy M, McGuire M. Pituitary apoplexy in the setting of COVID-19 infection: A case report. Am J Emerg Med. 2021. 47: e1-329
22. Lewis A, Frontera J, Placantonakis DG, Lighter J, Galetta S, Balcer L. Cerebrospinal fluid in COVID-19: A systematic review of the literature. J Neurol Sci 202;. 421: 117316
23. Lou JJ, Movassaghi M, Gordy D, Olson MG, Zhang T, Khurana MS. Neuropathology of COVID-19 (neuroCOVID): Clinicopathological update. Free Neuropathol. 2021. 2: 2
24. Matschke J, Lütgehetmann M, Hagel C, Sperhake JP, Schröder AS, Edler C. Neuropathology of patients with COVID-19 in Germany: A post-mortem case series. Lancet Neurol. 2020. 19: 919-29
25. Mazzeffi MA, Chow JH, Tanaka K. COVID-19 associated hypercoagulability: Manifestations, mechanisms, and management. Shock. 2021. 55: 465-71
26. Mukerji SS, Solomon IH. What can we learn from brain autopsies in COVID-19?. Neurosci Lett. 2021. 742: 135528
27. Muthukumar N. Pituitary apoplexy: A comprehensive review. Neurol India. 2020. 68: S72-8
28. Nielsen EH, Jørgensen JO, Bjerre P, Andersen M, Andersen C, Feldt-Rasmussen U. Acute presentation of craniopharyngioma in children and adults in a Danish national cohort. Pituitary. 2013. 16: 528-35
29. Oldfield EH, Merrill MJ. Apoplexy of pituitary adenomas: The perfect storm. J Neurosurg. 2015. 122: 1444-9
30. Post KD. Pituitary apoplexy: Is it one entity?. World Neurosurg. 2014. 82: 608-9
31. Santos CD, Filho LM, Santos CA, Neill JS, Vale HF, Kurnutala LN. Pituitary tumor resection in a patient with SARS-CoV-2 (COVID-19) infection. A case report and suggested airway management guidelines. Braz J Anesthesiol. 2020. 70: 165-70
32. Shahjouei S, Naderi S, Li J, Khan A, Chaudhary D, Farahmand G. Risk of stroke in hospitalized SARSCoV-2 infected patients: A multinational study. EBioMedicine. 2020. 59: 102939
33. Solomon IH, Normandin E, Bhattacharyya S, Mukerji SS, Keller K, Ali AS. Neuropathological features of Covid-19. N Engl J Med. 2020. 383: 989-92
34. Tan SK, Seow CJ, Tan E, Chau YP, Dalan R. Pituitary apoplexy secondary to thrombocytopenia due to dengue hemorrhagic fever: A case report and review of the literature. Endocr Pract. 2014. 20: e58-64
35. Teixeira JC, Lavrador J, Simão D, Miguéns J. Pituitary apoplexy: Should endoscopic surgery be the gold standard?. World Neurosurg. 2018. 111: e495-9
36. Workman AD, Welling DB, Carter BS, Curry WT, Holbrook EH, Gray ST. Endonasal instrumentation and aerosolization risk in the era of COVID-19: Simulation, literature review, and proposed mitigation strategies. Int Forum Allergy Rhinol. 2020. 10: 798-805
37. Xie J, Covassin N, Fan Z, Singh P, Gao W, Li G. Association between hypoxemia and mortality in patients with COVID-19. Mayo Clin Proc. 2020. 95: 1138-47
38. Zhang S, Zhang J, Wang C, Chen X, Zhao X, Jing H. COVID19 and ischemic stroke: Mechanisms of hypercoagulability (Review). Int J Mol Med. 2021. 47: 21
39. Zhou Z, Kang H, Li S, Zhao X. Understanding the neurotropic characteristics of SARS-CoV-2: From neurological manifestations of COVID-19 to potential neurotropic mechanisms. J Neurol. 2020. 267: 2179-84
40. Zhu W, Huang X, Zhao H, Jiang X. A COVID-19 patient who underwent endonasal endoscopic pituitary adenoma resection: A case report. Neurosurgery. 2020. 87: E140-6
41. Zoli M, Milanese L, Faustini-Fustini M, Guaraldi F, Asioli S, Zenesini C. Endoscopic endonasal surgery for pituitary apoplexy: Evidence on a 75-case series from a tertiary care center. World Neurosurg. 2017. 106: 331-8