- Department of Neurosurgery, Hospital Nacional Guillermo Almenara Irigoyen, La Victoria, Lima, Peru
- Department of Neurosurgery, Clínica Internacional, San Borja, Lima, Peru
- Department of Anatomical Pathology, Hospital Nacional Guillermo Almenara Irigoyen, La Victoria, Lima, Peru
Correspondence Address:
John Vargas-Urbina, Department of Neurosurgery, Hospital Nacional Guillermo Almenara Irigoyen, La Victoria, Lima, Peru.
DOI:10.25259/SNI_636_2024
Copyright: © 2025 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: John Vargas-Urbina1,2, Raúl Martinez-Silva1, Giuseppe Rojas-Panta1, Gabriel Ponce-Manrique1, Jerson Flores-Castillo1, William Anicama-Lima3. Unusual brain metastasis from colon cancer. 03-Jan-2025;16:5
How to cite this URL: John Vargas-Urbina1,2, Raúl Martinez-Silva1, Giuseppe Rojas-Panta1, Gabriel Ponce-Manrique1, Jerson Flores-Castillo1, William Anicama-Lima3. Unusual brain metastasis from colon cancer. 03-Jan-2025;16:5. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13313
Abstract
Background: Brain metastases due to colorectal cancer correspond to 3–5% of all brain metastases. The prognostic factors are based on age, functional status, and single metastasis. Its management is multidisciplinary, with poor prognosis despite the management.
Case Description: A case of a 64-year-old male presented with symptoms of headache, disorientation, and nausea. The enhanced magnetic resonance image showed a mass in the right frontal horn of the lateral ventricle, contrast enhancing, with irregular borders, but defined, without restriction in diffusion-weighted images, associated with obstructive hydrocephalus. The investigations in search of a primary neoplasm were negative. A ventricular endoscopic approach was performed, with total resection of the lesion. Four months later, he developed a bowel obstruction with surgical management to control the primary, followed by chemotherapy and radiotherapy, with a current survival longer than 1 year.
Conclusion: Brain metastases due to colorectal cancer are rare, and usually, when diagnosed, there are already pulmonary and hepatic metastases. Multidisciplinary management is recommended, where surgical management can be included in selected cases with controlled systemic disease, good functional condition, and single metastasis.
Keywords: Brain metastasis, Cerebral ventricle neoplasms, Colon cancer, Neoplasms unknown primary, Brain cancer
INTRODUCTION
Brain metastases from colorectal cancer are 3–5% of all brain metastases but are very rare, even though colorectal cancer is the third most common cancer in both men and women.[
Due to his scarce frequency, there is no protocol for the management of this type of patient, but studies performed determined the prognostic factors that are age under 65 years old, scale Eastern Cooperative Oncology Group (ECOG) of 0 or 1, single metastasis, <3 lines of previous chemotherapy and Karnofsky performance status (KPS) >70.[
In addition, it is not known the optimal treatment, but most studies suggest multimodal management, where radiotherapy and chemotherapy alone do not show a good response, and surgical management is the one that has shown the greatest survival, even >37 weeks, as long as the patient remains in good physical state with systemic controlled disease.[
This is why a rare case is presented. Brain metastases from colorectal cancer where the initial investigations did not find a primary neoplasm underwent multimodal treatment and carried a survival of >1 year to the time of this manuscript.
CASE DESCRIPTION
A 64-year-old male patient was admitted with a 2-week history of headache, disorientation, and nausea without vomiting. The examination revealed a Glasgow Coma Scale (GCS) of 14 points without additional neurological signs. The contrast-enhanced brain magnetic resonance imaging (MRI) showed a mass at the right frontal horn of the lateral ventricle adjacent to the foramen of Monro that generates a dilation of that horn; it is isointense on T1, contrast-enhancing, with irregular but defined borders, isointense on T2, which does not restrict diffusion [
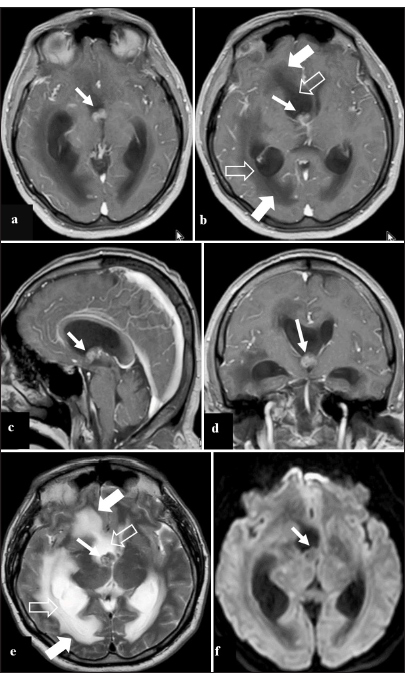
Figure 1:
Contrast-enhanced brain magnetic resonance imaging preoperative. (a) Contrast-enhanced T1 image in the axial section that shows a mass in the right frontal horn (arrow), which seems to obstruct the ipsilateral foramen of Monro. (b) Contrast-enhanced T1 image in axial view, 5 mm above image a, which shows the same tumor (thin arrow); furthermore, it shows ventricular dilation (empty arrow) with transependymal edema (thick arrow). (c) Contrast-enhanced T1 image in sagittal view shows the tumor in the right foramen of Monro (arrow). (d) Contrast-enhanced T1 image in coronal view, which evidences the tumor in the lower edge of the right frontal horn (arrow) close to the ipsilateral foramen of Monro. (e) T2 image shows a tumor in the right frontal horn, isointense (arrow thin), associated with ventricular dilation (empty arrow) and transependymal edema (thick arrow). (f) Diffusion-weighted image shows no restriction of the tumor of the right frontal horn (arrow).
A contrast-enhanced thoracoabdominopelvic computed tomography (CT) was negative for primary neoplasms, and tumor markers were also negative. It was decided to operate endoscopically, with the use of a handmade endoport, where we saw a gray-brownish, hypervascularized tumor adjacent to the foramen of Monro, with defined borders at the anterior and medial plane, with insertion in the lateral and posterior region, as well as in the floor of the ventricle [
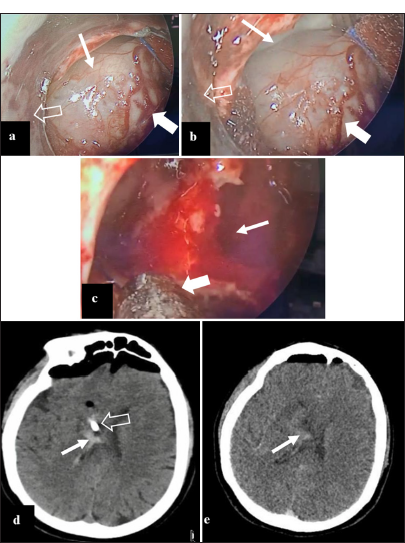
Figure 2:
(a) Intraoperative image, showing a brownish gray tumor (thin arrow), hypervascularized (thick arrow), by endoscopic view using a handmade endoport (empty arrow). (b) Intraoperative endoscopic image with a handmade endoport (empty arrow), where the same tumor is shown (thick arrow) with defined borders (thin arrow) that allow distinguishing the tumor from the medial wall of the right frontal horn of the lateral ventricle. (c) Intraoperative image with partial resection tumor with moderate bleeding (thin arrow), where the resection continued with aspiration (thick arrow). (d) Nonenhanced brain computed tomography (CT) on the 1st postoperative day, absence of tumor, with little bleeding in the surgical field is shown (thin arrow), also the distal end of the external ventricular catheter is seen (empty arrow). (e) Enhanced brain CT on the 5th postoperative day, without external ventricular shunt, where there is no evidence of contrast-enhancing lesions, with little bleeding in the surgical field (arrow).
The result of the pathological anatomy showed that it was a metastatic mucosecretory type adenocarcinoma, cytokeratin 20 (CK20) positive, B catenin positive, cytokeratin 7 (CK7) negative, thyroid transcription factor - 1 (TTF1) negative, glial fibrillary acidic protein (GFAP) negative, neuron-specific enolase (NSE) negative, Ki67 90-95%, suggestive of the colon as a possible primary [
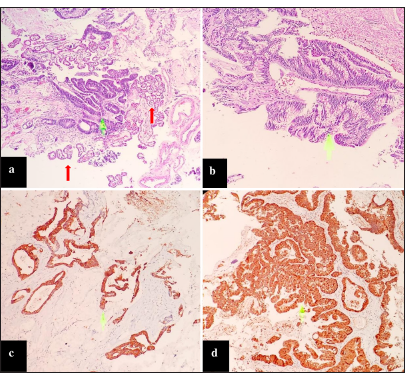
Figure 3:
Microscopic description: (a) Proliferation of malignant glandular formations (green arrow) immersed in a mucinous matrix. Note the presence of choroid plexuses with benign characteristics (red arrows). Hematoxylin and eosin (HE) ×4. (b) Malignant glandular epithelium, with mucinous cytoplasm. HE ×10. (c) Malignant cells were positive for CK20. HE ×4. (d) They were also positive for β catenin. HE ×10.
Four months after surgery, the patient was admitted with symptoms of a 10 kg loss in the past 2 months, and 1 day before admission, he presented constipation, abdominal distension, nausea, and fecaloid vomiting, for which an abdominal X-ray was performed, showing air-fluid levels, a new contrast-enhanced abdominopelvic CT scan was performed which revealed a neoplastic lesion in the transverse colon. In addition, a contrast-enhanced brain MRI was performed during the hospitalization, which showed a recurrence/residual tumor in the right frontal horn of the lateral ventricle, as well as another metastasis in the IV ventricle, without hydrocephalus [
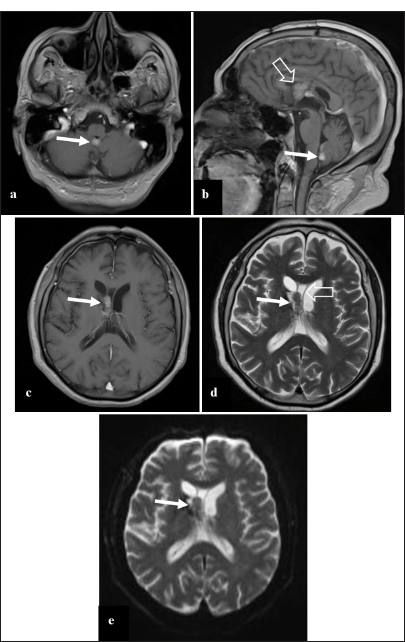
Figure 4:
Contrast-enhanced brain magnetic resonance imaging in the 4th postoperative month. (a) Contrast-enhanced T1 image in axial view shows a new metastasis, nodular, contrast-enhancing in the inferior border of the IV ventricle (arrow). (b) Contrast-enhanced T1 image in sagittal view shows the metastasis in the IV ventricle (arrow); in addition, the lesion recurrence in the right front horn is seen (empty arrow). (c) Contrast-enhanced T1 image in axial view shows the recurrence of the contrast-enhancing right frontal horn tumor (arrow), without associated ventricular dilation. (d) T2 image shows a recurrence of the right frontal horn tumor, isointense (thin arrow), that seems to invade the cavum vergae (empty arrow) and not the former surgical field. (e) Diffusion-weighted image shows no signal restriction (arrow).
He underwent a right hemicolectomy extended to transverse for a tumor of the proximal transverse colon plus ileotransverse anastomosis end-to-side plus the closure of the transverse colon stump plus cavity lavage and two laminar drains were placed (right parietocolic and rectovesical pouch). After tolerating oral administration without complications, he was discharged 15 days after surgery.
The pathological anatomy of the colon showed an invasive mucinous adenocarcinoma with moderate differentiation of the transverse colon that invades pericolonic adipose tissue, ileocecal valve without evidence of neoplasia, appendix without evidence of neoplasia, negative surgical margins, and three positive lymph nodes out of 23.
He began chemotherapy 6 months after brain surgery and 2 months after abdominal surgery, with an ECOG scale of 1, with the capecitabine and oxaliplatin regimen, of which he received eight cycles in 6 months, having an ECOG of 2 at the end of it.
One year after cranial surgery, he received stereotaxic radiosurgery with a linear accelerator with a total dose of 27 Gy in 3 doses, with 95% isodose. After this, the patient is with ECOG 2, with thoracoabdominopelvic enhanced CT without evidence of recurrent disease or liver or lung metastases. At the time of writing this article, the patient had survived 15 months since cranial surgery, and the possibility of starting monotherapy with capecitabine had been considered.
DISCUSSION
Brain metastases occur in 20–30% of cancer patients, and brain metastases from colorectal cancer account for 3–5% of all brain metastases.[
It is known that distant metastasis is the most common form of colorectal cancer recurrence, with the liver being the first with 79%, followed by lung, bone, or central nervous system, all with <6%,[
In general, at the time of diagnosis of a metastatic brain tumor from colorectal cancer, 85% of patients have lung metastasis, and 50–76% have liver involvement. They are rarely diagnosed before the primary one and even more, so that they do not have involvement of other organs,[
Brain metastasis from colorectal cancer on MRI is usually well-defined lesions, isointense on T1 and hyperintense on T2, with ring contrast-enhancement with central necrosis and with or without restricted diffusion, with moderate perilesional edema, and rarely has calcifications.[
Three possible routes of hematogenous dissemination of colorectal cancer are proposed to generate brain metastasis. The first is the spread through the rectal venous plexus toward the inferior vena cava, then to the lungs, and finally to the brain without passing through the liver; the second is the spread toward Batson’s vertebral plexus without passing through the liver or lungs; and the third is the spread to the portal veins, passing to the liver, then to the lungs and finally to the brain.[
Carcinoembryonic antigen (CEA) is a useful marker in colorectal neoplasm follow-up, but its value in terms of neurological compromise has not been demonstrated in series. It is known that CEA can be useful in leptomeningeal metastases but not in brain metastases[
Roussille et al. found in their study that the factors associated with a good prognosis were age under 65 years, ECOG scale of 0 or 1, single metastasis, and <3 lines of prior chemotherapy.[
The response to radiotherapy is much lower than its counterparts caused by lung or breast cancer, without variations in response in the different radiotherapy schemes, with a study that showed a response in only two patients of the 11 treated, with a 9-week survival average.[
However, stereotactic radiosurgery is a good option, with studies showing local disease control in 77.8% of patients after 1 year of follow-up and an average survival rate of 7 months; Chernov concluded that it is as effective as whole-brain radiotherapy.[
On the other hand, in the case series, surgical resection is the one that has had the best survival rate, even >37 weeks, and generally, the patients are in good physical and general condition, with controlled systemic disease.[
Recent studies show that multimodal management, with a combination of radiotherapy, chemotherapy, or targeted therapy, improves the prognosis of patients with nonoperable colorectal cancer.[
CONCLUSION
Brain metastases from colorectal carcinoma are rare, and when they occur, they usually already have lung or liver metastases. In the case of single metastasis with controlled systemic disease and good performance status, they are the best candidates for first-line surgical management followed by complementary treatment (chemotherapy, radiotherapy, or targeted therapy). In other cases, multimodal management is the best and should be personalized according to each case.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Borgelt B, Gelber R, Kramer S, Brady LW, Chang CH, Davis LW. The palliation of brain metastases: Final results of the first two studies by the radiation therapy oncology group. Int J Radiat Oncol Biol Phys. 1980. 6: 1-9
2. Bresalier RS, Karlin DA. Meningeal metastasis from rectal carcinoma with elevated cerebrospinal fluid carcinoembryonic antigen. Dis Colon Rectum. 1979. 22: 216-7
3. Cascino TL, Leavengood JM, Kemeny N, Posner JB. Brain metastases from colon cancer. J Neurooncol. 1983. 1: 203-9
4. Chahine G, Ibrahim T, Felefly T, El-Ahmadie A, Freiha P, El-Khoury L. Colorectal cancer and brain metastases: An aggressive disease with a different response to treatment. Tumori J. 2019. 105: 427-33
5. Chernov M, Hayashi M. Radiosurgery for brain metastases of colorectal cancer. Br J Radiol. 2022. 95: 20210179
6. Clifford Schold S, Wasserstrom WR, Fleisher M, Schwartz MK, Posner JB. Cerebrospinal fluid biochemical markers of central nervous system metastases. Ann Neurol. 1980. 8: 597-604
7. Deck MD, Messina AV, Sackett JF. Computed tomography in metastatic disease of the brain. Radiology. 1976. 119: 115-20
8. DeVita VT, Lawrence TS, Rosenberg SA, editors. DeVita, Hellman, and Rosenberg’s cancer: Principles and practice of oncology. Philadelphia, PA: Wolters Kluwer-Lippincott Williams & Wilkins; 2008. p.
9. Di Muzio B, editors. Metastatic colorectal adenocarcinoma - lung and brain metastases. Radiopaedia.org. 2015. p. Available from https://radiopaedia.org/cases/metastatic-colorectaladenocarcinoma-lung-and-brain-metastases [Last accessed on 2024 Jul 30]
10. Finkelmeier F, You SJ, Waidmann O, Wolff R, Zeuzem S, Bähr O. Bevacizumab in combination with chemotherapy for colorectal brain metastasis. J Gastrointestinal Cancer. 2016. 47: 82-8
11. Hammound MA, McCutcheon IE, Elsouki R, Schoppa D, Patt YZ. Colorectal carcinoma and brain metastasis: Distribution, treatment, and survival. Ann Surg Oncol. 1996. 3: 453-63
12. Knipe H, Salam H, editors. Cerebral metastasis-colorectal carcinoma. Radiopaedia.org. 2010. p. Available from https://radiopaedia.org/cases/cerebral-metastasis-colorectal-carcinoma [Last accessed on 2024 Dec 06]
13. Li W, Wang T, Zhu Y, Yu H, Ma L, Ding Y. Brain metastasis from colorectal cancer: Treatment, survival, and prognosis. Medicine (Baltimore). 2022. 101: e30273
14. Lu X, Cai Y, Xia L, Ju H, Zhao X. Treatment modalities and relative survival in patients with brain metastasis from colorectal cancer. Biosci Trends. 2019. 13: 182-8
15. Mege D, Sans A, Ouaissi M, Iannelli A, Sielezneff I. Brain metastases from colorectal cancer: Characteristics and management. ANZ J Surg. 2018. 88: 140-5
16. Olson RM, Perencevich NP, Malcolm AW, Chaffey JT, Wilson RE. Patterns of recurrence following curative resection of adenocarcinoma of the colon and rectum. Cancer. 1980. 45: 2969-74
17. Roussille P, Auvray M, Vansteene D, Lecomte T, Rigault E, Maillet M. Prognostic factors of colorectal cancer patients with brain metastases. Radiother Oncol. 2021. 158: 67-73
18. Ruelle A, Macchia G, Gambini C, Andrioli G. Unusual appearance of brain metastasis from adenocarcinoma of colon. Neuroradiology. 1986. 28: 375
19. Simsek M, Deveci M. Cerebellar metastasis of colon cancer at diagnosis: A very rare case. J Cancer Res Ther. 2022. 18: 493
20. Temple DF, Ledesma EJ, Mittelman A. Cerebral metastases. From adenocarcinoma of the colon and rectum. N Y State J Med. 1982. 82: 1812-4
21. Winston KR, Walsh JW, Fischer EG. Results of operative treatment of intracranial metastatic tumors. Cancer. 1980. 45: 2639-45
22. Yamada K, Bremer AM, West CR, Ghoorah J, Park HC, Takita H. Intra-arterial BCNU therapy in the treatment of metastatic brain tumor from lung carcinoma. A preliminary report. Cancer. 1979. 44: 2000-7