- Department of Neurosurgery, George Washington University Hospital, Washington, United States
Correspondence Address:
Ryan Neill, Department of Neurosurgery, George Washington University Hospital, Washington, United States.
DOI:10.25259/SNI_789_2024
Copyright: © 2025 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Ryan Neill, Peter Harris, Lekhaj Chand Daggubati. Bone cement versus bone flap replacement: A comparative meta-analysis of posterior fossa craniotomy complications. 31-Jan-2025;16:25
How to cite this URL: Ryan Neill, Peter Harris, Lekhaj Chand Daggubati. Bone cement versus bone flap replacement: A comparative meta-analysis of posterior fossa craniotomy complications. 31-Jan-2025;16:25. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13360
Abstract
Background: Posterior fossa surgeries are often performed to treat infratentorial pathologies, such as tumors that increase intracranial pressure. Posterior fossa craniotomy has been shown to decrease the incidence of postoperative complications and morbidity compared to craniectomy. More recently, the use of bone cement in posterior fossa craniotomies has been implemented, but there is limited comparative postoperative data of this technique to more commonly used bone flap replacement. This study aims to address this information gap through a meta-analysis comparing the incidence of postoperative cerebrospinal fluid leakage and other complications when utilizing bone cement versus bone flap replacement in posterior fossa craniotomies.
Methods: Following a literature review, search parameters for a systematic review were identified and relevant studies were sorted based on selection criteria to be included in the meta-analysis. Data analysis was performed in R studio and Microsoft Excel software. Targeted complications for analysis include cerebrospinal fluid (CSF) leakage, pseudomeningocele formation, and infection. Pooled estimates and odds ratios for dichotomous outcomes were calculated with corresponding 95% confidence intervals, and findings were translated into illustrative tables and figures.
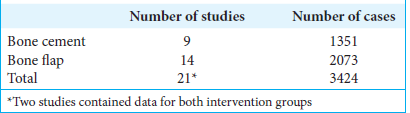
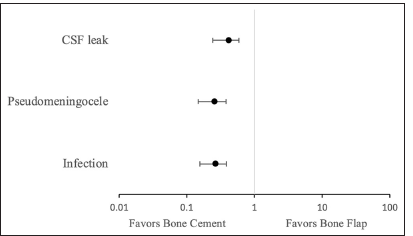
Results: Twenty-one articles were included in a systematic review, nine studies using bone cement and thirteen using bone flap (two studies reported data for both groups). With bone flap replacement, CSF leakage was 8.36% (95% confidence interval [CI] 5.89–10.86%), pseudomeningocele formation was 9.22% (95% CI 4.82–13.62%), and infection was 6.85% (95% CI 4.05–9.65%). With bone cement usage, CSF leakage was 3.47% (95% CI 2.37–4.57%), pseudomeningocele formation was 2.43% (95% CI 1.23–3.63%), and infection was 1.85% (95% CI 0.75–2.95%). The odds ratio of CSF leak, pseudomeningocele formation, and infection was 0.39 (95% CI 0.229–0.559), 0.25 (95% CI 0.137–0.353), and 0.26 (95% CI 0.149–0.363), respectively, with the use of bone cement compared to craniotomy.
Conclusion: Outcomes demonstrated in this meta-analysis revealed an overall decreased incidence of postoperative complications rates of CSF leak, pseudomeningocele formation, and infection when using bone cement compared to bone flap in posterior fossa craniotomies. Our study suggests that bone cement use is safe and effective in posterior fossa surgery. Future studies should further assess the comparative outcomes of these techniques.
Keywords: Bone cement, Bone flap, Meta-analysis, Neurosurgery, Posterior fossa craniotomy
INTRODUCTION
Posterior fossa craniotomies are required for a variety of infratentorial pathologies. Infratentorial mass lesions can impact the posterior fossa by blocking spinal fluid flow, leading to increased intracranial pressure.[
There are several approaches to addressing posterior fossa cranial defects. Originally, the bone flap was replaced with titanium plates and screws;[
Bone cement is a biomaterial commonly utilized in orthopedic surgery where a powder phase and liquid phase are mixed and then either injected to harden or molded and implanted into the body.[
The use of bone cement (frequently HBC) in cranioplasties can integrate an implanted titanium mesh overlay with the HBC prosthesis.[
The distinction between complication rates in postoperative outcomes when using a bone flap replacement technique versus bone cement in closing posterior fossa craniotomies is still underreported in the literature. This study aims to address this information gap through a comparative meta-analysis of these two posterior fossa craniotomy surgical approaches by comparing the incidence of postoperative CSF leak and other complications when implementing bone cement versus bone flap replacement.
MATERIALS AND METHODS
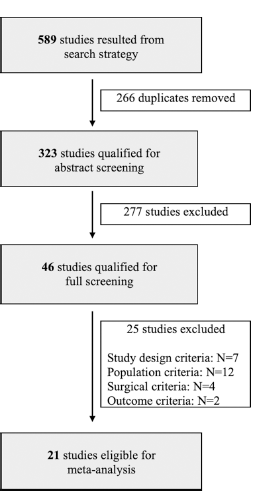
A literature review was conducted to assess the current state and gaps of knowledge concerning bone cement utilization versus bone flap replacement in posterior fossa craniotomies. Following Preferred Reporting Items for Systematic Reviews and meta-analysis guidelines, search parameters for the systematic review were identified, and relevant studies were sorted based on selection criteria for the meta-analysis [
RESULTS
A total of 589 studies were initially obtained through the search criteria, and 266 were removed as duplicates. Of the 323 studies screened, 21 proved viable to be included in the meta-analysis. Of those included, 9 studies reported data regarding bone cement[
In the bone flap group, the rate of CSF leak, pseudomeningocele formation, and infection were each not reported in one separate instance of the fourteen studies. In the bone cement group, pseudomeningocele formation was not reported in two of the nine studies; CSF leak and infection were reported in all. Corresponding patient cases (n) with unreported data were not included in the corresponding incidence summation total when data were not recorded.
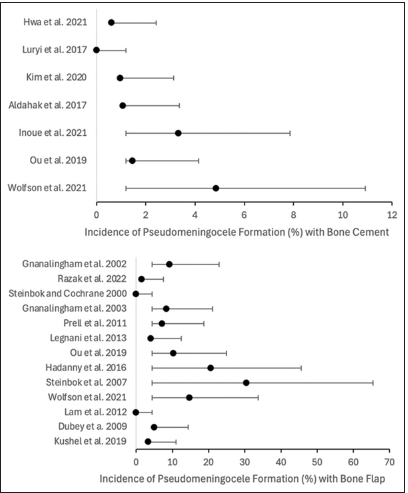
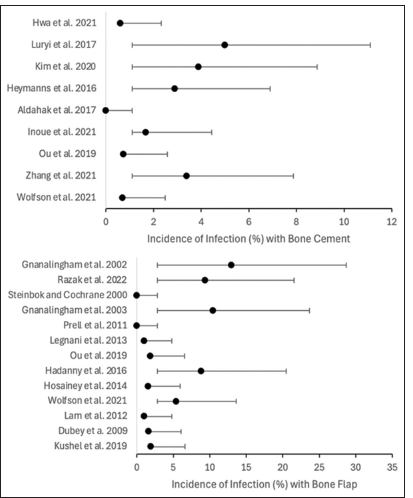
Summative analysis regarding the use of bone flap revealed the following overall incidence rates: CSF leakage was 8.36% (95% CI 5.89–10.86%), pseudomeningocele formation was 9.22% (95% CI 4.82–13.62%), and infection was 6.85% (95% CI 4.05–9.65%). Comparatively, the use of bone cement revealed the following overall incidence rates: CSF leakage was 3.47% (95% CI 2.37–4.57%), pseudomeningocele formation was 2.43% (95% CI 1.23–3.63%), and infection was 1.85% (95% CI 0.75–2.95%) [
The odds ratio (OR) indicates that bone cement usage is 39.41% as likely to experience CSF leak (OR 0.39 with 95% CI 0.165 [0.229–0.559]), 25.52% as likely to experience pseudomeningocele formation (OR 0.25 with 95% CI 0.108 [0.137–0.353]), and 25.59% as likely to experience infection compared to when bone flaps are used (OR 0.26 with 95% CI 0.107 [0.149–0.363]).
The overall incidence of CSF leakage with bone cement was 3.47% and 8.36% with bone flap [
DISCUSSION
A total of 3424 cases (1351 using bone cement and 2073 using bone flap techniques) from 21 studies (9 for bone cement and 14 for bone flap; 2 studies provided data for both techniques) were included in the final analysis. Statistical examination in this meta-analysis showed that the overall incidence of CSF leakage in posterior fossa craniotomies was 8.36% with bone flap and 3.47% with bone cement; pseudomeningocele formation was 9.22% with bone flap and 2.43% with bone cement; infection was 6.85% with bone flap and 1.85% with bone cement. These results underline the relevance of the craniotomy closure technique in posterior fossa craniotomy complications.
Odds ratio calculations demonstrated that bone cement usage resulted in outcomes 39.41% as likely to experience CSF leak, 25.52% as likely to experience pseudomeningocele formation, and 25.59% as likely to experience infection than when the bone flap is used. The reduced complication rates of CSF leakage and pseudomeningocele formation may be owed to the closer reapproximation of the cranial defect and less dead space for potential fluid to pass through or accumulate.[
In addition to increased morbidity and mortality, complications from posterior fossa surgery prolong hospital stay, increase/prolong inpatient and outpatient antibiotic usage, delay treatment of malignancies, increase return to the OR, and increase costs.[
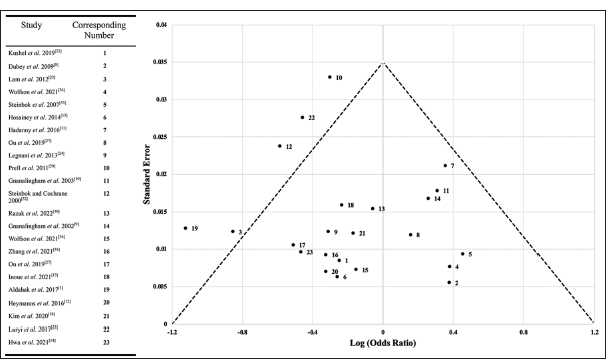
This meta-analysis is subject to several limitations. Most importantly, the studies included are heterogeneous in the metric; they are primarily evaluating. Relevant data were extracted upstream of the various studies’ analyses, but the specific parameters of individual studies may shape the data they present, such as which complications are reported in a specific study and how those complications were defined by those reporting and grouping case values. There may be variability in the degree of complications reported that are grouped, along with the potential for cofounders in the type of posterior fossa craniotomy and comorbidities. These confounders were considered in the study design and mitigated in selection criteria primarily by removing low-quality case series (i.e., noninstitutional reports, comorbid cases, surgical approach variability). The calculated heterogeneity index (I2) in this meta-analysis is 24.54%. In addition, more studies have been published regarding bone flap than bone cement due to the time course and other factors, generating greater sample sizes for bone flap data and posing the potential of selection bias due to data availability. In addition to limited data, continuous advancements in operative care outside of the specific interventions analyzed in this study may have impacted reported complication rates. Samples utilizing cement may be less impacted by complications due to other confounding considerations of care advancements unrelated to the surgical technique, such as various technological instruments and pharmacologic agents. These factors, coupled with potential selection bias regarding what clinical data are published, present a variety of limitations that require further study to validate the findings in this meta-analysis. A funnel plot comparing the studies included in this meta-analysis demonstrates reasonable distribution [
In summary, this meta-analysis reports an overall incidence of CSF leakage in posterior fossa craniotomies as 8.36% with bone flap and 3.47% with bone cement (39.41% as likely with bone cement), pseudomeningocele formation 9.22% with bone flap and 2.43% with bone cement (25.52% as likely with bone cement), and infection 6.85% with bone flap and 1.85% with bone cement (25.59% as likely with bone cement), underlining the relevance of craniotomy closure technique for complications.
CONCLUSION
The use of bone cement cranioplasty compared to bone flap replacement in posterior fossa craniotomies demonstrated a significantly decreased incidence of postoperative CSF leak, pseudomeningocele formation, and infection. Future prospective studies are needed to assess potential mechanisms for the improved outcomes further, provide direct comparative outcomes of these techniques, and limit intrinsic confounding factors within a meta-analysis to validate the findings of bone cement cranioplasty superiority in this meta-analysis.
Disclosures
The authors have no personal, financial, or institutional interests in any of the drugs, materials, or devices described in this manuscript.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent is not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
Acknowledgments
The authors would like to acknowledge the patients composing the 3424 cases analyzed in this study, as well as the authors of the studies included in this meta-analysis.
References
1. Aldahak N, Dupre D, Ragaee M, Froelich S, Wilberger J, Aziz KM. Hydroxyapatite bone cement application for the reconstruction of retrosigmoid craniectomy in the treatment of cranial nerves disorders. Surg Neurol Int. 2017. 8: 115
2. Alhantoobi MR, Kesserwan MA, Khayat HA, Lawasi M, Sharma S. Rates of cerebrospinal fluid leak and pseudomeningocele formation after posterior fossa craniotomy versus craniectomy: A systematic review and meta-analysis. Surg Neurol Int. 2023. 14: 140
3. Anetsberger S, Mellal A, Garvayo M, Diezi M, Perez MH, Beck Popovic M. Predictive factors for the occurrence of perioperative complications in pediatric posterior fossa tumors. World Neurosurg. 2023. 172: e508-16
4. Beer-Furlan A, Vellutini EA, Gomes MQ, Cardoso AC, Prevedello LM, Todeschini AB. Approach selection and surgical planning in posterior cranial fossa meningiomas: How I do it. J Neurol Surg B Skull Base. 2019. 80: 380-91
5. Bray HN, Sappington JM. A review of posterior fossa lesions. Mo Med. 2022. 119: 553-8
6. Chen K, Liang W, Zhu Q, Shen H, Yang Y, Li Y. Clinical outcomes after cranioplasty with titanium mesh, polyetheretherketone, or composite bone cement: A retrospective study. J Craniofac Surg. 2023. 34: 2246-51
7. Crawford K, Berrey BH, Pierce WA, Welch RD. In vitro strength comparison of hydroxyapatite cement and polymethylmethacrylate in subchondral defects in caprine femora. J Orthop Res. 1998. 16: 715-9
8. Dubey A, Sung WS, Shaya M, Patwardhan R, Willis B, Smith D. Complications of posterior cranial fossa surgery--an institutional experience of 500 patients. Surg Neurol. 2009. 72: 369-75
9. Gnanalingham KK, Lafuente J, Thompson D, Harkness W, Hayward R. Surgical procedures for posterior fossa tumors in children: does craniotomy lead to fewer complications than craniectomy?. J Neurosurg. 2002. 97: 821-6
10. Gnanalingham K, Lafuente J, Thompson D, Harkness W, Hayward R. MRI study of the natural history and risk factors for pseudomeningocoele formation following postfossa surgery in children. Br J Neurosurg. 2003. 17: 530-6
11. Hadanny A, Rozovski U, Nossek E, Shapira Y, Strauss I, Kanner AA. Craniectomy versus craniotomy for posterior fossa metastases: Complication profile. World Neurosurg. 2016. 89: 193-8
12. Heymanns V, Oseni AW, Alyeldien A, Maslehaty H, Parvin R, Scholz M. Sandwich wound closure reduces the risk of cerebrospinal fluid leaks in posterior fossa surgery. Clin Pract. 2016. 6: 824
13. Hosainey SA, Lassen B, Helseth E, Meling TR. Cerebrospinal fluid disturbances after 381 consecutive craniotomies for intracranial tumors in pediatric patients. J Neurosurg Pediatr. 2014. 14: 604-4
14. Hwa TP, Luu N, Henry LE, Naples JG, Kaufman AC, Brant JA. Impact of reconstruction with hydroxyapatite bone cement on CSF leak rate in retrosigmoid approach to vestibular schwannoma resection: A review of 196 cases. Otol Neurotol. 2021. 42: 918-22
15. Inoue T, Shitara S, Shima A, Goto Y, Fukushima T. Double collagen matrix grafting for dural closure in microvascular decompression: An alternative use of autologous fascial grafting. Acta Neurochir (Wien). 2021. 163: 2395-401
16. Jagannathan S, Krovvidi H. Anaesthetic considerations for posterior fossa surgery. Contin Educ Anaesth Crit Care Pain. 2014. 14: 202-6
17. Kassam A, Horowitz M, Carrau R, Snyderman C, Welch W, Hirsch B. Use of Tisseel fibrin sealant in neurosurgical procedures: Incidence of cerebrospinal fluid leaks and cost-benefit analysis in a retrospective study. Neurosurgery. 2003. 52: 1102-5 discussion 1105
18. Kim KH, Park B, Byoun HS, Lim J, Kwon HJ, Choi SW. Ten-year experience of dural reconstruction using a collagen matrix inlay graft in posterior fossa surgery: A propensity score-matched study. World Neurosurg. 2020. 141: e383-8
19. Komang-Agung IS, Hydravianto L, Sindrawati O, William PS. Effect of polymethylmethacrylate-hydroxyapatite composites on callus formation and compressive strength in goat vertebral body. Malays Orthop J. 2018. 12: 6-13
20. Krishnan SS, Nigam P, Manuel A, Vasudevan MC. Bone sandwich closure technique for posterior fossa craniectomy. J Neurol Surg B Skull Base. 2020. 81: 8-14
21. Kurpad SN, Cohen AR. Posterior fossa craniotomy: An alternative to craniectomy. Pediatr Neurosurg. 1999. 31: 54-7
22. Kushel Y, Danilov G, Tekoev A, Cheldiev B, Strunina Y. A single-center retrospective descriptive cohort study of 211 pediatric patients: Cerebrospinal fluid leakage after fourth ventricle tumor resection. World Neurosurg. 2019. 129: e171-76
23. Lam FC, Kasper E. Augmented autologous pericranium duraplasty in 100 posterior fossa surgeries-a retrospective case series. Neurosurgery. 2012. 71: ons302-7
24. Legnani FG, Saladino A, Casali C, Vetrano IG, Varisco M, Mattei L. Craniotomy vs. craniectomy for posterior fossa tumors: A prospective study to evaluate complications after surgery. Acta Neurochir (Wien). 2013. 155: 2281-6
25. Luryi AL, Bulsara KR, Michaelides EM. Hydroxyapatite bone cement for suboccipital retrosigmoid cranioplasty: A single institution case series. Am J Otolaryngol. 2017. 38: 390-3
26. Muzumdar D. Postoperative pneumoventricle following posterior fossa tumor surgery in sitting position: Plugging the aqueduct. J Pediatr Neurosci. 2020. 15: 1-4
27. Ou C, Chen Y, Mo J, Wang S, Gai S, Xing R. Cranioplasty using polymethylmethacrylate cement following retrosigmoid craniectomy decreases the rate of cerebrospinal fluid leak and pseudomeningocele. J Craniofac Surg. 2019. 30: 566-70
28. Shirvan AR, Nouri A, Wen C, editors. Structural biomaterials: Properties, characteristics and selection. Structural polymer biomaterial 12.8.1.4 Bone cements.. United Kingdom: Woodhead Publishing; 2021. p. 395-439
29. Prell J, Scheller C, Alfieri A, Scheller C, Alfieri A, Rampp S. Midline-craniotomy of the posterior fossa with attached bone flap: Experiences in paediatric and adult patients. Acta Neurochir (Wien). 2011. 153: 541-5
30. Razak AA, Youshani AS, Krishnan R, D’urso PI. Safety and feasibility of posterior fossa neurosurgery in the supine position-A UK series from a large centre. J Clin Neurosci. 2022. 101: 150-3
31. Slot EM, van Baarsen KM, Hoving EW, Zuithoff NP, van Doormaal TP. Cerebrospinal fluid leakage after cranial surgery in the pediatric population-a systematic review and meta-analysis. Childs Nerv Syst. 2021. 37: 1439-47
32. Steinbok P, Cochrane DD. Posterior fossa craniotomy: An alternative to craniectomy: an alternative to craniectomy. Pediatr Neurosurg. 2000. 32: 110
33. Steinbok P, Singhal A, Mills J, Cochrane DD, Price AV. Cerebrospinal fluid (CSF) leak and pseudomeningocele formation after posterior fossa tumor resection in Children: A retrospective analysis. Childs Nerv Syst. 2007. 23: 171-4 discussion 175
34. Thapa AJ, Lei BX, Zheng MG, Li ZJ, Liu ZH, Deng YF. The surgical treatment of posttraumatic skull base defects with cerebrospinal fluid leak. J Neurol Surg B Skull Base. 2018. 79: 205-16
35. Vaishya R, Chauhan M, Vaish A. Bone cement. J Clin Orthop Trauma. 2013. 4: 157-63
36. Wolfson DI, Magarik JA, Godil SS, Shah HM, Neimat JS, Konrad PE. Bone cement cranioplasty reduces cerebrospinal fluid leak rate after microvascular decompression: A single-institutional experience. J Neurol Surg B Skull Base. 2021. 82: 556-61
37. Zaben M, Richards A, Merola J, Patel C, Leach P. Surgical site infection in paediatric posterior fossa surgery: Does pathology matter?. Childs Nerv Syst. 2021. 37: 1859-61
38. Zhang L, Galaiya D, Jackson CM, Tamargo RJ, Lim M, Carey J. Bone cement internal auditory canal reconstruction to reduce CSF leak after vestibular schwannoma retrosigmoid approach. Otol Neurotol. 2021. 42: e1101-5