- Department of Neurosurgery, Hospital Italiano de Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina
- Department of Anesthesiology, Hospital Italiano de Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina
Correspondence Address:
Florencia Casto, Department of Neurosurgery, Hospital Italiano de Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina.
DOI:10.25259/SNI_791_2024
Copyright: © 2025 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Florencia Casto1, Miguel Villaescusa1, Ezequiel Jungberg1, Tomas Saavedra Azcona1, Delfina Lanusse2, Pedro Plou1, Matteo Baccanelli1, Pablo Ajler1. Efficacy of topical hemostatic agents in neurosurgery: An experimental study in a rat model. 25-Apr-2025;16:148
How to cite this URL: Florencia Casto1, Miguel Villaescusa1, Ezequiel Jungberg1, Tomas Saavedra Azcona1, Delfina Lanusse2, Pedro Plou1, Matteo Baccanelli1, Pablo Ajler1. Efficacy of topical hemostatic agents in neurosurgery: An experimental study in a rat model. 25-Apr-2025;16:148. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13524
Abstract
BackgroundFew studies have compared different topical hemostatic agents in live models or brain tissue, and their doses are not standardized. Little is known about the combined use of these different elements in terms of efficacy and safety, especially in neurosurgery. The objective of this study was to evaluate the efficacy of different topic hemostatic agents used in daily neurosurgical practice in an experimental animal model study.
MethodsA group of 42 Wistar rats was used. A stereotaxic frame was fixed, and coordinates were determined to locate the bregma. A 3 mm hole was drilled with a bone-profile burr on each side of the midline. A stylet was inserted into the brain to create the defect and induce bleeding. The rats were randomly divided into seven groups, with each group assigned a hemostatic agent. Hemostasis time and control time on the opposite side were measured.
ResultsHemostasis was achieved after an average of 1 82 s in the group treated with Beriplast, making it the hemostatic agent that stopped the bleeding the fastest. The control time was an average of 40, 14 s. Compared with the negative control, all the agents resulted in significantly better hemostasis (P
ConclusionA reduction in postoperative bleeding positively impacts annual morbidity and mortality rates, hospitalization time, and hospital bed turnover. Understanding the efficacy and safety of different hemostatic agents will enable surgeons to optimize intraoperative hemostasis, thereby achieving better postoperative outcomes and increased patient safety.
Keywords: Coagulation, Experimental study, Hemostasis time, Rat model, Topical hemostatic
INTRODUCTION
Hemostasis can be described as a tightly regulated process that ensures that blood flow is maintained through the vascular system while a thrombotic response to tissue injury takes place. Controlling hemostasis is a critical stage in any surgical intervention and involves complex interactions among the vascular wall, platelets, and the balance between coagulation and fibrinolysis.[
MATERIALS AND METHODS
Surgical protocol and planning
This study used a group of 42 adult Wistar rats (weighing between 400 and 600 g, aged 3–4 months). This protocol was evaluated and approved by the Institutional Committee for the Care and Use of Laboratory Animals of our hospital, and animal handling was performed in accordance with their policies and the Guide for the Care and Use of Laboratory Animals. Informed consent was not needed. This study was conducted on rat brains because their circulation and brain anatomy are similar to those of humans.[
Experimental phase
The animal was weighed, and general anesthesia was administered with a dose of 10 mg/kg xylazine and 80 mg/kg ketamine intramuscularly under the care of a specialized veterinarian. After induction, the reflexes were checked to ensure complete anesthesia. The rats were randomly distributed into seven groups, each assigned a topical hemostatic agent. The hemostatic agent, if necessary, was prepared immediately before the procedure by the surgeon [
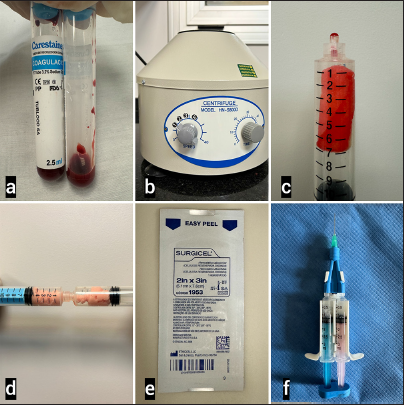
Regenerated oxidized cellulose (Surgicel, Absorbable Hemostatic, Ethicon Inc., New Brunswick, New Jersey): available in sizes of 3 × 2 in, 8 × 4 in, 14 × 2 in, or 2 × 1.5 in, mesh forms that can be cut as needed. A key advantage is that it is immediately ready for use, unlike Surgiflo and Beriplast, which need to be prepared on the operating table. On contact with blood, it forms a brown gelatinous substance that aids in clot formation, serving as a hemostatic adjunct in controlling local bleeding. If left in the surgical bed, it will be reabsorbed within 7–14 days, depending mainly on the amount of product and degree of blood saturation. It should not be used in bone foramina, confined bony areas, the spinal cord, or the optic chiasm and nerve. It can expand spontaneously and may exert undue pressure.[ Fluid Porcine Gelatin Combined with Human Thrombin (Surgiflo, Hemostatic Matrix, Ethicon Inc., New Brunswick, New Jersey): available as an 8 mL syringe of fluid porcine gelatin, with human thrombin in a powder vial (2,000 IU) mixed with 2 mL of sterile water, forming a single activated component in a syringe with an applicator tip. After the required amount is applied, the excess is irrigated. It has a reabsorption time of 4–6 weeks. This product should also be removed from the site when used around or near bone holes, confined bony areas, the spinal cord, and/or the optic nerve and chiasm, as it may enlarge and cause nerve damage.[ Fibrinogen combined with human thrombin (Beriplast, CSL Behring, Germany): widely used in various neurosurgical procedures, including aneurysm clipping, tumor excision, dural closure to prevent cerebrospinal fluid leakage, neurorrhaphy, and bleeding control during spinal surgery.[ PRP: This is a fraction of plasma that is obtained from autologous blood after centrifugation. It contains a high concentration of platelets and growth factors that are actively secreted by the platelets. PRP is rich in certain proteins and agents that act on cellular adhesion (such as fibrin, fibronectin, and vitronectin), providing structural support for processes such as cellular migration and tissue growth. PRP has effects on the extracellular matrix and stimulates tissue repair and regeneration.[
Figure 1:
Preparation of Hemostatic Agents. (a) Blood samples were obtained from the rats for centrifugation to obtain platelet-rich plasma (PRP). (b) Centrifuge used to obtain PRP. (c) Fluid porcine gelatin combined with human thrombin (Surgiflo hemostatic matrix, Ethicon Inc., New Brunswick, New Jersey), before preparation, combined with PRP. (d) The two components of Surgiflo were mixed to obtain the active compound. (e) Oxidized regenerated cellulose (Surgicel, absorbable hemostatic, Ethicon Inc., New Brunswick, New Jersey). (f) Fibrinogen combined with human thrombin (Beriplast, CSL Behring, Germany).
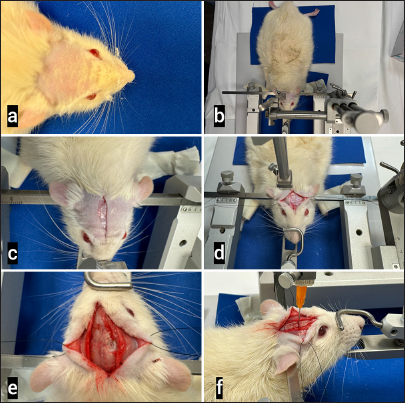
Figure 2:
Model for the Transcranial Approach in Animals. (a) The animal, previously anesthetized, was shaved. (b) It was positioned in a prone position and fixed using a stereotaxic system (Stoelting, Illinois, USA) with attachments to both external ear canals and a third fixation at the oral level. Total immobilization was confirmed. (c) A sagittal incision was made on the skin. (d) The periosteum was dissected, and the bilateral flaps were reflected to expose the skull. The sagittal and coronal sutures were observed. (e) After the craniotomy point was marked with the stereotaxic system and coordinates, a trephination hole was done with a diamond drill until the dura mater was exposed. The dura was then opened with a number 11 scalpel blade. (f) Finally, a needle was inserted to a depth of 2 mm to induce bleeding using the stereotaxic system. A randomly assigned hemostatic agent was applied, and the hemostasis time was measured with a stopwatch. Steps (e and f) were repeated on the contralateral side without a hemostatic agent as a control time.
Table 1:
Classification of hemostatic and topical sealant agents on the basis of Emilia et al.[
The following groups were established in which the study rats were randomly designated:
Group 1: Regenerated oxidized cellulose (Surgicel) 0.1 cm3 Group 2: Fluid porcine gelatin combined with human thrombin (Surgiflo) 0.1 mL Group 3: Fibrinogen combined with human thrombin (Beriplast) 0.1 mL Group 4: Surgicel 0.1 cm3 + PRP Group 5: Beriplast 0.1 mL + PRP Group 6: Surgiflo 0.1 mL + PRP Group 7: PRP 0.1 mL.
In each case, the hemostasis time (defined as the time in seconds between incision and cessation of visible spontaneous bleeding) was measured with a stopwatch, and the number of applications needed to achieve hemostasis was recorded. Any residual hemostatic material was irrigated. The procedure was repeated on the contralateral side of the same animal without the application of any hemostatic agent, which served as a control. Complications and/or adverse reactions during or after surgery were recorded.
Termination
All euthanasia techniques must ensure the absence of pain or distress; the method should be painless and stress-free, facilitating rapid unconsciousness and death.[
Statistical analysis
Longitudinal data analysis was performed through a one-way analysis of variance (ANOVA). Specific comparisons between all of the treatment arms were conducted through ANOVA to evaluate significant differences. The homogeneity of the variances was assessed at this stage. To offer clearer insight into the trends, individual profile plots were created. To establish the specific differences between groups, post hoc tests with multiple paired comparisons were used. t-tests were used to assess if there was a statistically significant difference between the means of two paired groups. This approach allowed not only an examination of general patterns but also the identification of potential variations in individual responses and differences between treatment groups and negative controls. Values of P < 0.05 were considered statistically significant, with 2 standard deviations included in the measurements obtained. All the data are expressed as the means ± standard deviations.
RESULTS
Efficacy evaluation
Hemostasis was achieved after all the hemostatics were applied for 1 minute [
DISCUSSION
As more surgical procedures are performed through minimally invasive incisions, tools that can reduce bleeding by inducing blood coagulation, sealing vessels, or adhering tissues are becoming increasingly important.[
Takizawa et al. used 16 rabbits to test the hemostatic efficacy of gelatin sponges alone (Gelfoam) and oxidized cellulose alone (Surgicel), both alone and combined with fibrin adhesive (Bolheal).[
Sabab et al. used 90 rats to study topical hemostatics.[
Since the active agents are directly involved in the final stages of the coagulation cascade and bypass the initial enzymatic steps, other components of the coagulation cascade may be dysfunctional or scarce without significantly affecting the local hemostatic efficacy of the products.[
Limitations
Although the administration of each hemostatic agent was randomized for the animal, there was no blinding of the operator due to the nature of the surgical intervention. In addition, measuring visible bleeding cessation is not a precise endpoint for hemostasis because this process is better assessed with imaging studies, such as a CT scan. The doses of the products used in this trial were fixed for the small size of the brain lesion. Further studies using different doses for increasing extents of brain injury should be carried out as an attempt to correlate quantity to extension. The use of a small animal model is another limitation of this study, as there are differences in vascular size, flow, coagulation, and pressure parameters compared with those of humans. The Wistar rat has a heart rate of 300–500 beats/min, hematocrit of 36–54, platelet count of 600 × 103 uL (± 150 × 103), and systolic blood pressure of approximately 114 ± 2 mmHg. As vital signs in humans significantly differ, the response to hemostatic agents during surgery must also differ. In this study, vital signs were not controlled continuously during the intervention, so the findings were not correlated with these normal physiological variables. Further investigations using other models are needed to confirm these findings, which will serve as a starting point until protocols for standard use in humans are proposed. Future research could focus on the application of these hemostatics in the context of known coagulation disorders or concomitant antiplatelet or anticoagulant therapy. Another limitation of this study is that the survival rate and behavior of the rats were not assessed postoperatively, as in some studies mentioned previously [
CONCLUSION
Reducing the time that a surgeon needs to manage intraoperative bleeding decreases operative times and optimizes human resources. A reduction in postoperative hemorrhage incidence positively impacts annual morbidity and mortality, hospital stay duration, and bed turnover. Understanding the efficacy and safety of different hemostatics will allow surgeons to optimize intraoperative hemostasis, achieving better postoperative results and greater patient safety.
Ethical approval
The research/study approved by the Institutional Review Board at CICUAL- IMTIB, Institutional Committee for the Care and Use of Laboratory Animals, Italian Hospital of Buenos Aires, number 023/24, dated May 21, 2024.
Declaration of patient consent
Patient’s consent was not required as there are no patients in this study.
Financial support and sponsorship
Publication of this article was made possible by the James I. and Carolyn R. Ausman Educational Foundation.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Altun I. An experimental study of histopathologic effects of hemostatic agents used in spinal surgery. World Neurosurg. 2016. 90: 147-53
2. American Veterinary Medical Associati, editors. AVMA Guidelines for the Euthanasia of animals: 2013 edition. Schaumburg: American Veterinary Medical Association; 2013. p. 6-7
3. Brandenberg G, Leibrock LG, Shuman R, Malette WG, Quigley H. Chitosan: A new topical hemostatic agent for diffuse capillary bleeding in brain tissue. Neurosurgery. 1984. 15: 9-13
4. Cecyn MN, Abrahao KP. Where do you measure the Bregma for rodent stereotaxic surgery?. IBRO Neurosci Rep. 2023. 15: 143-8
5. Emilia M, Luca S, Francesca B, Luca B, Paolo S, Giuseppe F. Topical hemostatic agents in surgical practice. Transfus Apher Sci. 2011. 45: 305-11
6. Ereth MH, Schaff M, Ericson EF, Wetjen NM, Nuttall GA, Oliver WC. Comparative safety and efficacy of topical hemostatic agents in a rat neurosurgical model. Neurosurgery. 2008. 63: 369-72 discussion 372
7. Gabay M. Absorbable hemostatic agents. Am J Health Syst Pharm. 2006. 63: 1244-53
8. Gasteratos K, Paladino JR, Akelina Y, Mayer HF. Superiority of living animal models in microsurgical training: Beyond technical expertise. Eur J Plast Surg. 2021. 44: 167-76
9. Khazipov R, Zaynutdinova D, Ogievetsky E, Valeeva G, Mitrukhina O, Manent JB. Atlas of the postnatal rat brain in stereotaxic coordinates. Front Neuroanat. 2015. 9: 161
10. Masoudi M, Wiseman J, Wiseman SM. A contemporary systematic review of the complications associated with SURGICEL. Expert Rev Med Devices. 2023. 20: 741-52
11. Rajiv S, Harding M, Bassiouni A, Jardeleza C, Drilling A, James C. The efficacy and safety of chitosan dextran gel in a burr hole neurosurgical sheep model. Acta Neurochir (Wien). 2013. 155: 1361-6 discussion 1366
12. Rodríguez Flores J, Palomar Gallego MA, Torres GarcíaDenche J. Platelet-rich plasma: Biology and applications in maxillofacial surgery and facial aesthetics. Rev Esp Cirug Oral Maxilofac. 2012. 34: 8-17
13. Sabab A, Vediappan RS, Finnie J, McAdam CJ, Jukes A, Vreugde S. Efficacy and safety of novel beta-chitin patches as haemostat in rat vascular and neurosurgical model. Front Surg. 2022. 9: 830364
14. Sabel M, Stummer W. The use of local agents: Surgicel and surgifoam. Eur Spine J. 2004. 13: S97-101
15. Samudrala S. Topical hemostatic agents in surgery: A surgeon’s perspective. AORN J. 2008. 88: S2-11
16. Sirieix D, Chemla E, Castier Y, Massonnet-Castel S, Fabiani JN, Baron JF. Comparative study of different biological glues in an experimental model of surgical bleeding in anesthetized rats: platelet-rich and-poor plasma-based glue with and without aprotinin versus commercial fibrinogen-based glue. Ann Vasc Surg. 1998. 12: 311-6
17. Takizawa K, Okazaki D, Takegawa Y, Koga Y, Sagata M, Michishita K. Evaluation of the hemostatic effect of a combination of hemostatic agents and fibrin glue in a rabbit venous hemorrhage model. BMC Neurol. 2021. 21: 270