- Department of Neurosurgery, Anhembi Morumbi University, São José dos Campos, São Paulo, Brazil
- Department of Neurosurgery, Federal University of Rio de Janeiro, Rio de Janeiro, Brazil
- Department of Medicine, Barão de Mauá University Center, Faculty of Medicine, Ribeirão Preto, São Paulo, Brazil
- Department of Medicine and Life Sciences, Pontifical Catholic University of Paraná, Curitiba, Brazil
- Department of Neurosurgery, Neurosolution Clinic, São José dos Campos, Brazil
- Department of Neurosurgery, Nucleo Oscar Freire, Salvador, São Paulo, Brazil
Correspondence Address:
Luis Flavio Fabrini Paleare, Pontifical Catholic University of Paraná, Curitiba, Paraná, Brazil.
DOI:10.25259/SNI_179_2025
Copyright: © 2025 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Murilo dos Santos Mancilha1, Filipi Fim Andreão2, Bárbara de Ávila Costa Januário1, Filipe Virgilio Ribeiro3, Matheus Nepomuceno Fernandes1, Luis Flavio Fabrini Paleare4, Bruno Pasqualino Aguilar da Silva5, Marcelo Medeiros Felippe5, Danilo Gomes Quadros6. Craniocervical hypertrophic pachymeningitis. 16-May-2025;16:179
How to cite this URL: Murilo dos Santos Mancilha1, Filipi Fim Andreão2, Bárbara de Ávila Costa Januário1, Filipe Virgilio Ribeiro3, Matheus Nepomuceno Fernandes1, Luis Flavio Fabrini Paleare4, Bruno Pasqualino Aguilar da Silva5, Marcelo Medeiros Felippe5, Danilo Gomes Quadros6. Craniocervical hypertrophic pachymeningitis. 16-May-2025;16:179. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13562
Abstract
Background: Hypertrophic pachymeningitis (HP) is a rare neurological disorder characterized by dural thickening. Here, we discuss the diagnosis and surgical management of a 38-year-old whose myelopathy was attributed to dorsally compressive HP extending from the lower cerebellar fossa to C3.
Case Description: A 38-year-old male with Sjögren’s syndrome presented with cervical pain, upper limb paresis, dysphagia, and left-sided tongue/palate paralysis. The cervical magnetic resonance (MR) showed a dorsally compressive lower cerebellar fossa to C3 lesion. When the biopsy revealed HP, and once conservative treatment failed, the patient successfully underwent a posterior surgical decompression, lesion debulking, and craniocervical fusion.
Conclusion: Cervical HPs should be diagnosed early on MR, and those with significant myelopathy, aggressively surgically treated.
Keywords: Case report, Craniocervical junction, Dural thickening, Hypertrophic pachymeningitis, Meningitis
INTRODUCTION
Hypertrophic pachymeningitis (HP) rarely involves thee spine. Chronic HP, due to progressive thickening/fibrosis of the dura mater, can contribute to brain/spinal cord compression.[
CASE DESCRIPTION
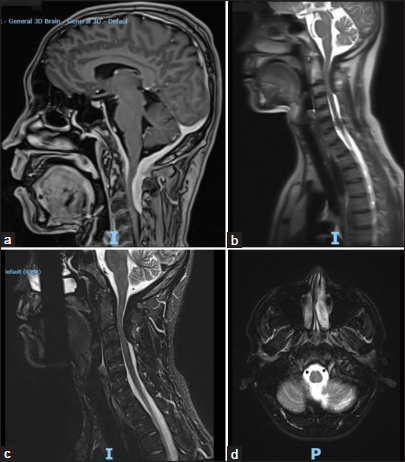
A 38-year-old male patient presented with 4 months of increased difficulty speaking due to left-sided tongue/palate paralysis a progressive quadriparesis. The cervical MR imaging (MRI) showed an expansive/dorsally compressive dural broad-based posterior cranio-cervical junction lesion extending from the lower cerebellar fossa to the C3/C4 level. The dorsal dural lesion was 9 mm thick in its largest anteroposterior diameter and displaced the spinal cord anteriorly. Further, the cranial MRI revealed regular, concentric, and homogeneous thickening of the pachymeninges that extended from the anterior sella turcica along the entire ventral aspect of the brainstem [
Figure 1:
Preoperative magnetic resonance (a) sagittal T1-weighted spoiled gradient echo magnetic resonance imaging (MRI) for high-resolution anatomical imaging, (b) localizing scan in three planes (axial, coronal, and sagittal), (c) short-tau inversion recovery MRI of T1 spine, highlighting soft tissues/edema, and (d) axial section of T2 level.
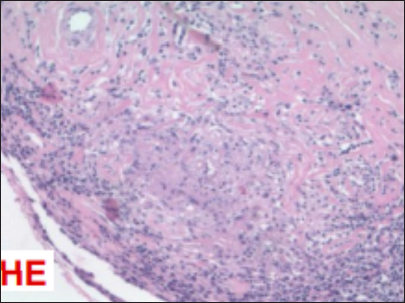
Biopsy and immunohistochemical analysis
The biopsy and immunohistochemical analyses of the lesion revealed chronic lymphoplasmacytic meningitis (i.e., focally granulomatous, without acid-fast bacilli or fungi on special stains). Cellular markers confirmed the presence of B and T lymphocytes and macrophages, suggesting a chronic inflammatory process. However, there was no evidence of a syphilitic infection, and no Langerhans cells were present [
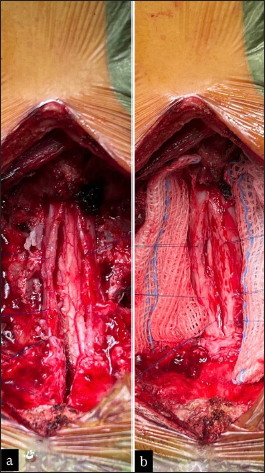
Craniocervical junction HP surgery
The patient failed conservative treatment and required a posterior fossa/C1–C3 craniectomy/laminectomy; it was performed under intraoperative neurophysiological monitoring using total intravenous anesthesia. At surgery, the HP lesion appeared as a thickening of the dura mater [
DISCUSSION
Nodular HP is a rare chronic inflammatory process that involves cranial and/or spinal dural fibrosis.[
CONCLUSION
HP rarely causes cranial/spinal dural thickening and brain/cord compression. Establishing the correct diagnosis with MR, obtaining biopsy confirmation of the HP lesion, and following with partial/gross total HP tumor resection is critical to optimize neurological improvement/recovery.
Ethical approval:
The Institutional Review Board approval is not required.
Declaration of patient consent:
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship:
Nil.
Conflicts of interest:
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation:
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
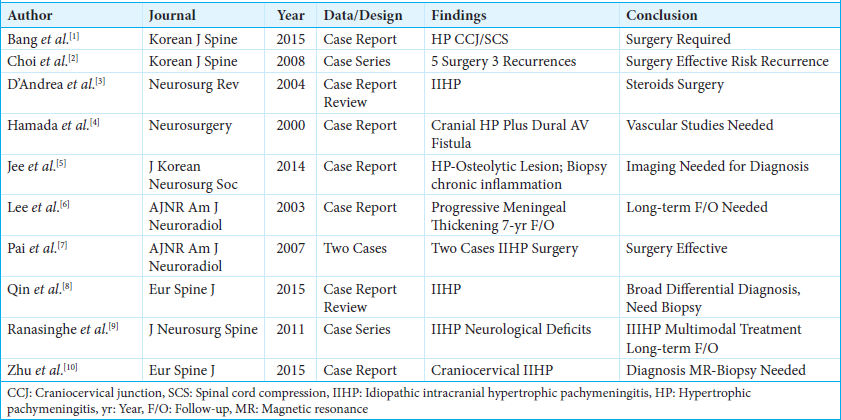
1. Bang JH, Cho KT, Kim EJ. Idiopathic hypertrophic pachymeningitis in the craniocervical junction. Korean J Spine. 2015. 12: 169-72
2. Choi HJ, Yoon DH, Lee DY, Ahn PG, Yang MS, Yi S. Rapid recurrence of spinal idiopathic hypertrophic pachymeningitis: A case report. Korean J Spine. 2008. 5: 211-4
3. D’Andrea G, Trillò G, Celli P, Roperto R, Crispo F, Ferrante L. Idiopathic intracranial hypertrophic pachymeningitis: Two case reports and review of the literature. Neurosurg Rev. 2004. 27: 199-204
4. Hamada J, Yoshinaga Y, Korogi Y, Ushio Y. Idiopathic hypertrophic cranial pachymeningitis associated with a dural arteriovenous fistula involving the straight sinus: Case report. Neurosurgery. 2000. 47: 1230-3
5. Jee TK, Lee SH, Kim ES, Eoh W. Idiopathic hypertrophic spinal pachymeningitis with an osteolytic lesion. J Korean Neurosurg Soc. 2014. 56: 162-5
6. Lee YC, Chueng YC, Hsu SW, Lui CC. Idiopathic hypertrophic cranial pachymeningitis: Case report with 7 years of imaging follow-up. AJNR Am J Neuroradiol. 2003. 24: 119-23
7. Pai S, Welsh CT, Patel S, Rumboldt Z. Idiopathic hypertrophic spinal pachymeningitis: Report of two cases with typical MR imaging findings. AJNR Am J Neuroradiol. 2007. 28: 590-2
8. Qin LX, Wang CY, Hu ZP, Zeng LW, Tan LM, Zhang HN. Idiopathic hypertrophic spinal pachymeningitis: A case report and review of literature. Eur Spine J. 2015. 24: S636-43
9. Ranasinghe MG, Zalatimo O, Rizk E, Specht CS, Reiter GT, Harbaugh RE. Idiopathic hypertrophic spinal pachymeningitis. J Neurosurg Spine. 2011. 15: 195-201
10. Zhu R, He Z, Ren Y. Idiopathic hypertrophic craniocervical pachymeningitis. Eur Spine J. 2015. 24: S633-5