- Department of Pediatric Neurosurgery, Santo Antonio Children’s Hospital, Santa Casa de Misericórdia de Porto Alegre,
- Neurosurgery, Hospital de Clínicas de Porto Alegre, Federal University of Rio Grande do Sul, Porto Alegre, Brazil.
- Departments of Pathology, School of Medicine, Hospital de Clínicas de Porto Alegre, Federal University of Rio Grande do Sul, Porto Alegre, Brazil.
Correspondence Address:
Cristina Birlem Bleil
Neurosurgery, Hospital de Clínicas de Porto Alegre, Federal University of Rio Grande do Sul, Porto Alegre, Brazil.
DOI:10.25259/SNI-237-2019
Copyright: © 2019 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Cristina Birlem Bleil, Jorge Wladimir Junqueira Bizzi, Andre Bedin, Francine Hehn de Oliveira, Ápio Cláudio Martins Antunes. Survival and prognostic factors in childhood medulloblastoma: A Brazilian single center experience from 1995 to 2016. 25-Jun-2019;10:120
How to cite this URL: Cristina Birlem Bleil, Jorge Wladimir Junqueira Bizzi, Andre Bedin, Francine Hehn de Oliveira, Ápio Cláudio Martins Antunes. Survival and prognostic factors in childhood medulloblastoma: A Brazilian single center experience from 1995 to 2016. 25-Jun-2019;10:120. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=9408
Abstract
Background: Medulloblastoma is the most common malignant brain tumor in the pediatric population. Despite prognosis improvement in the past two decades, one-third of the patients still remain incurable. New evidence suggests that medulloblastoma comprises four distinct entities; therefore, treatment de-escalation is required. The aim of this article is to evaluate epidemiological data from patients treated at our institution. The primary objective is to analyze overall survival (OS) and event-free survival (EFS) and the secondary objective is to identify prognostic factor from this cohort.
Methods: We retrospectively analyzed 69 patients who underwent surgical resection for medulloblastoma among 423 children from the tumor registry data bank of Santo Antônio Children’s Hospital from 1995 to 2016. Kaplan–Meier method and Cox regression analysis were used to identify OS, EFS, and prognostic factors.
Results: The 5-year OS and EFS rates found were 44.5% and 36.4%, respectively. The extent of resection and radiotherapy as adjuvant treatments was positively correlated to outcome while metastatic disease at diagnosis was negatively related to OS. Age younger than 3 years old did not have a worse outcome in our cohort.
Conclusion: Similar results to population-based studies were found, but we still face difficulties due to living in a developing country. In the near future, we look forward to new diagnostic techniques that will enable us to classify medulloblastomas according to molecular subgroups.
Keywords: Childhood medulloblastoma, Event-free survival, Gross total resection, Overall survival, Prognostic factors
INTRODUCTION
Medulloblastomas are the most common malignant tumor of the central nervous system (CNS) in children.[
These tumors occur in the posterior fossa and they grow into the IV ventricle or in the cerebellar hemisphere leading to obstructive hydrocephalus.[
Since 1969, medulloblastoma risk stratification has undergone new modifications, and Chang’s original system is currently still in use.[
Survival rates in medulloblastoma patients improved toward the end of the 90s. Craniospinal radiotherapy as an adjuvant treatment reached overall cure rates of around 70%–85%. This led to a reduction in the risk of death by approximately 30%.[
In this article, we reviewed our 21 years’ experience in diagnosing and treating medulloblastomas at a children’s hospital, in Southern Brazil, dedicated to the public national health system. The aim of our study was to evaluate epidemiological data on medulloblastoma population, determining the spectrum and frequency of the variants encountered as well as if the data correlate with the current literature. The analysis of overall survival (OS) and event-free survival (EFS) rates in our cohort will enable us to check our institution treatment results and to compare to scientific literature data. The secondary objective is the identification of prognostic factors for survival among the analyzed variables.
PATIENTS AND METHODS
We retrospectively analyzed patients presenting histological medulloblastoma diagnosis from the tumor registry data bank of Santo Antonio Children`s Hospital. Among 423 children operated on for brain tumors, we identified 69 patients with medulloblastoma, diagnosed and treated between January 1995 and June 2016; all were operated by the same surgeon (JWJB). Only those with complete medical records and follow-up were included. Exclusion criteria were supratentorial primitive neuroectodermal tumors (PNETs) and insufficient clinical data and follow-up information.
The present study was approved by the hospital’s Research Ethics Committee in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments, under registration number CAAE 40232214.5.0000.5327.
Patient data were collected only from patients records on the following variables: age, sex, symptoms, prediagnostic symptomatic interval (PDSI), presence of hydrocephalus, tumor location, surgical approach, surgical resection, need of definitive hydrocephalus treatment, tumor histology, metastatic disease, tumor relapse, postoperative complication, and late sequelae.
Patient risk stratification was defined according to Chang’s system.[
Adjuvant conventional radiotherapy was performed according to current protocols so that high-risk patients received craniospinal irradiation of 36 Gy and a posterior fossa boost to complete 54 Gy. Standard risk patients instead received 24 Gy to the neuroaxis in addition to a posterior fossa boost to complete 54 Gy. CT standard protocol consisted of eight doses, once a week, of vincristine in a dose of 1.5 mg/m2 during radiation therapy (RT). Subsequently, eight cycles of vincristine in a dose of 1.5 mg/m2, 75 mg/m2 of cisplatin, and also 75 mg/m2 of lomustine were administered. A variation of this protocol was also used, with cyclophosphamide instead of lomustine, and in certain cases, etoposide was associated with these two described protocols. High-risk patients received a high- risk protocol known as head start, with high doses of methotrexate, vincristine, etoposide, cisplatin, and cyclophosphamide in five cycles followed by autologous stem cell rescue.[
The statistical analysis was performed with IBM SPSS® Statistics version 2.1. Quantitative variables were described by mean and standard deviation and interquartile range, depending on the distribution of data. Categorical variables were described by absolute and relative frequencies. OS and EFS were estimated with the Kaplan–Meier method. For OS, time was defined as the interval from the date of surgery to the date of death for all causes, with censoring at the date of the latest follow-up visit for live patients. For EFS, time was the interval also from the date of surgery to the date of an event such as relapse or death, with censoring at the latest follow-up visit for live patients and progression-free patients. For the prognostic effect of the variables, multivariate Cox regression analysis was applied. The criteria for entry of the variables into the multivariate model was that they had P < 0.20 value in the bivariate analysis and/or being relevant according to literature. The statistical significance level adopted was 5%.
RESULTS
Among the 69 patients enrolled from January 1995 to June 2016, only 61 patients had complete information in medical records, though two were excluded because they were not confirmed as medulloblastomas. From the 59 patients, 36 were male and 23 were female, a rate of 1.5:1.0. The mean age in this cohort was 6 years old, ranging from 5 months to 13 years old.
All patients underwent surgery, with gross total resection achieved of 76.8%. The patient who only underwent a biopsy had M4 stage metastatic disease diagnosed at clinical presentation, with lung impairment. A second surgery was necessary for 17 patients due to local relapse or as a second-look surgery due to residual disease.
There were neither intraoperative nor surgical mortalities, considering this as death occurring within 30 days after the surgical procedure. Postoperative complications such as cerebrospinal fluid (CSF) leakage happened in only five cases. CSF increased cellularity was seen in 15 cases, and despite negative cultures, all of them were treated as meningitis. Posterior fossa syndrome was diagnosed in five (8.5%) patients. Tumor relapse occurred in 20 (34%) patients, in a mean time of 17 months, ranging from 5.5 to 39 months.
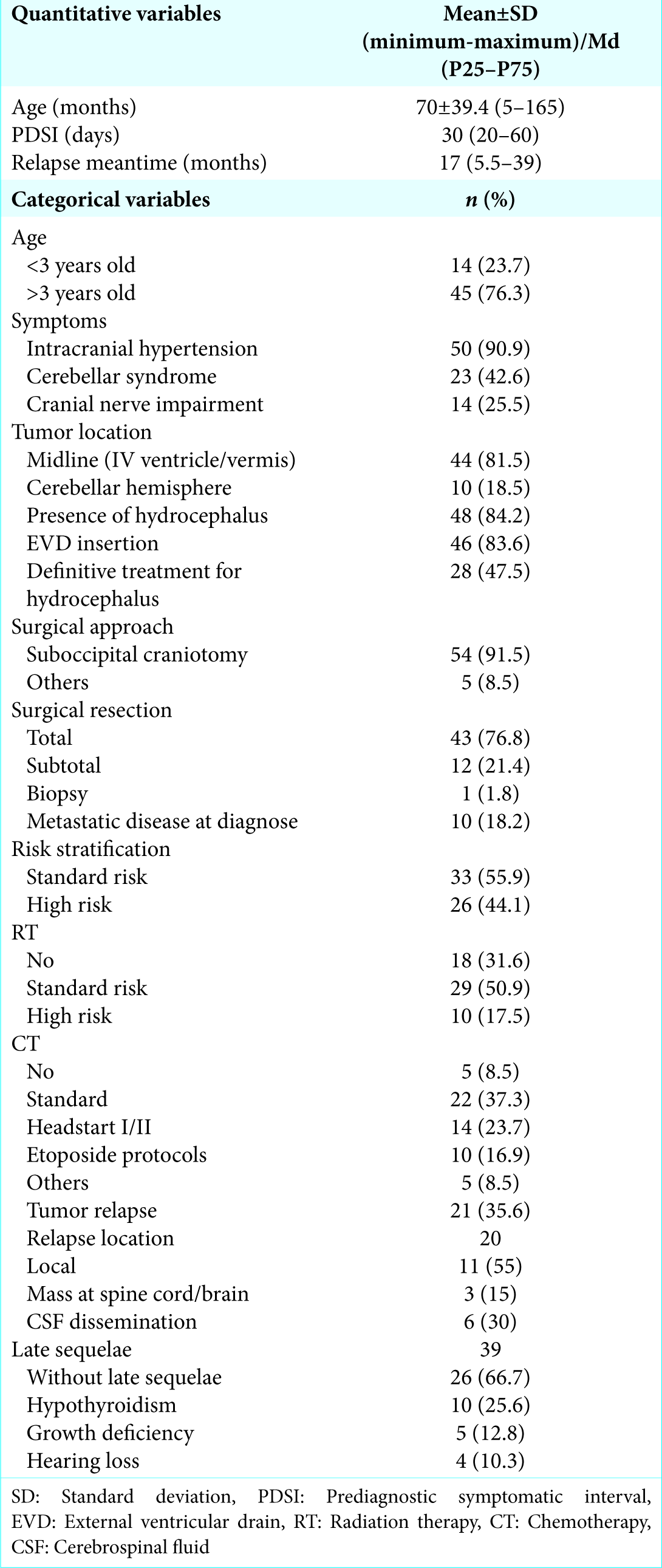
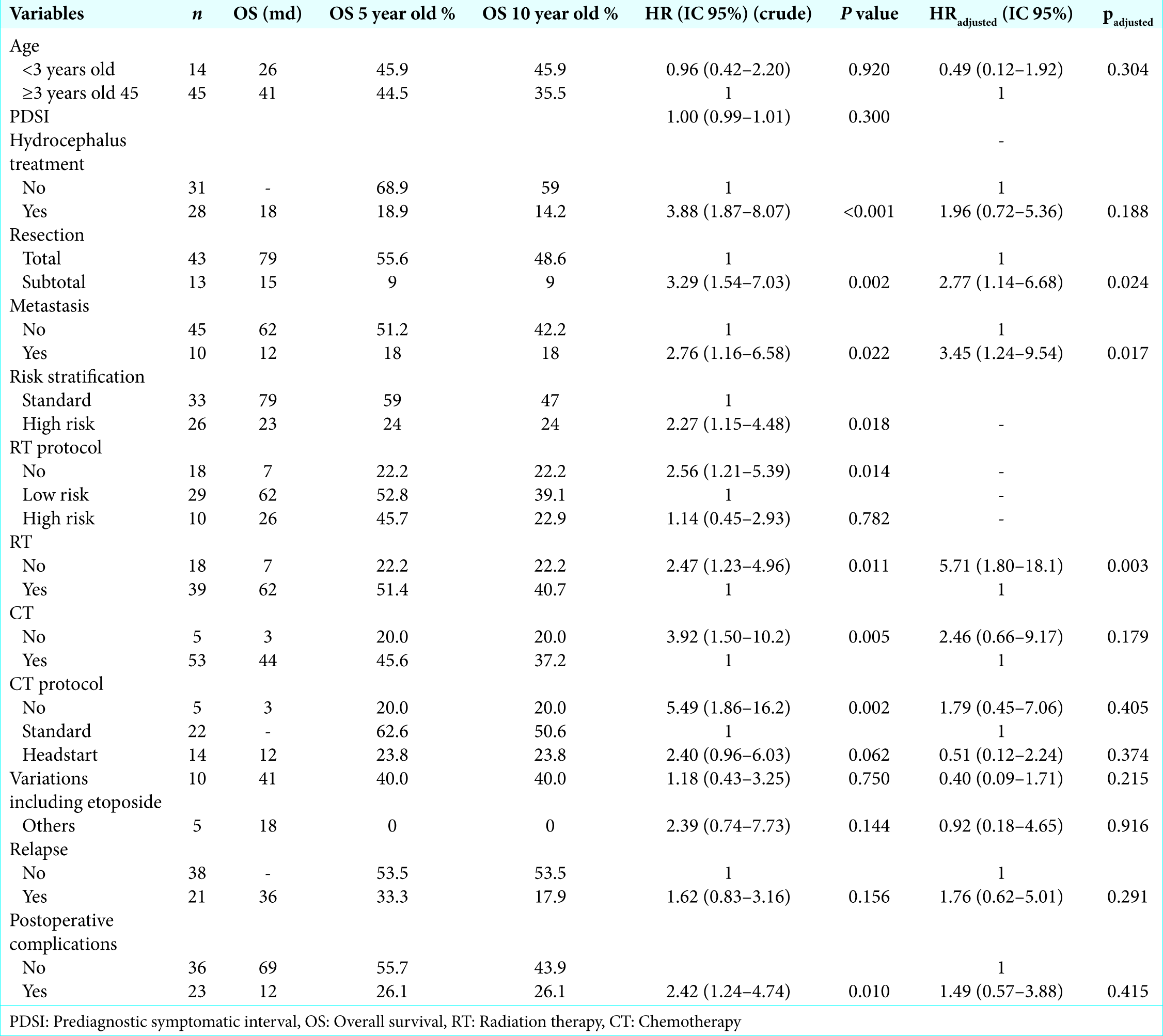
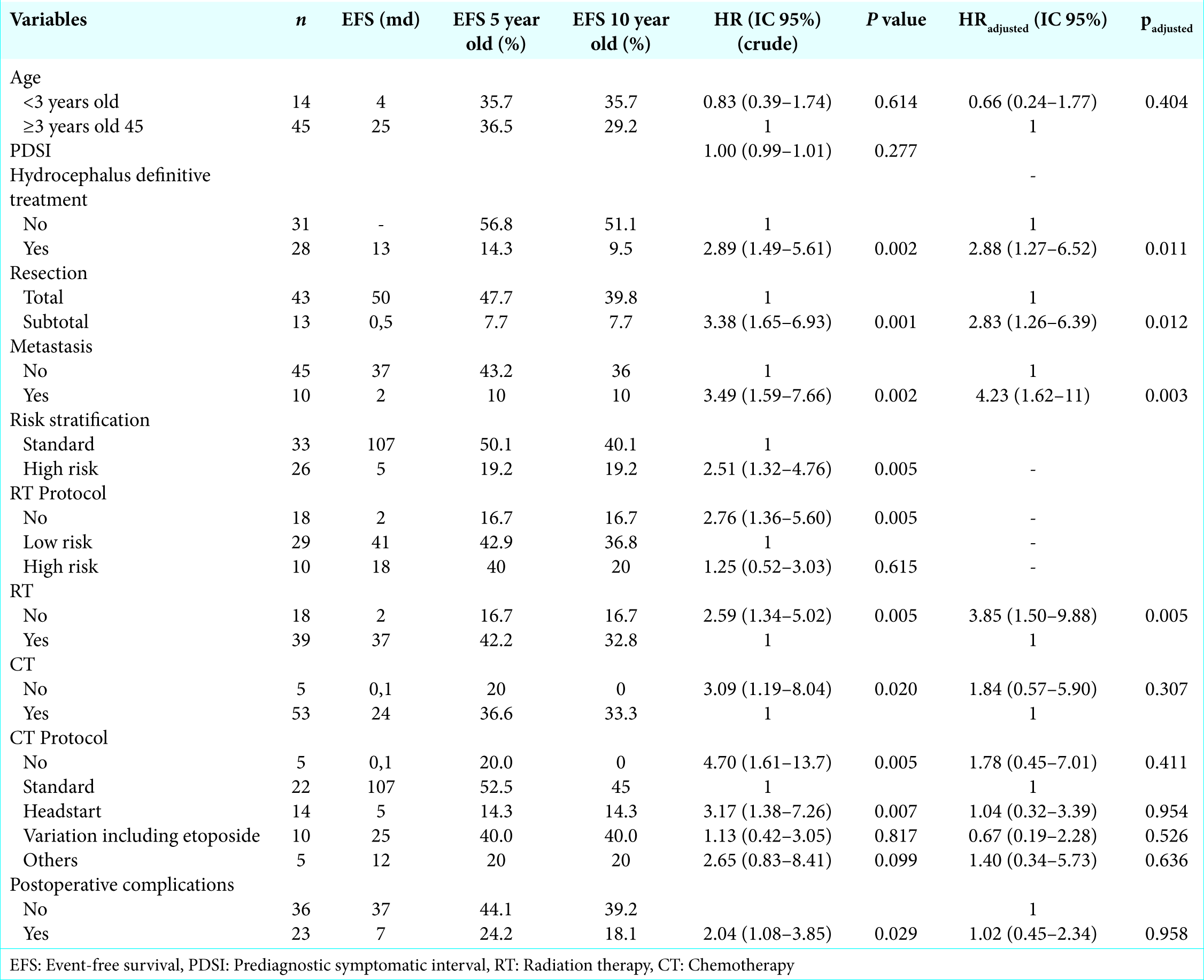
The 5-year OS and EFS were 44.5% and 36.4%, respectively, after a median follow-up time of 29 months, with an interquartile range of 10–79. The EFS and OS rates from this cohort according to the variables are shown in
Long-term survival according to treatment protocols
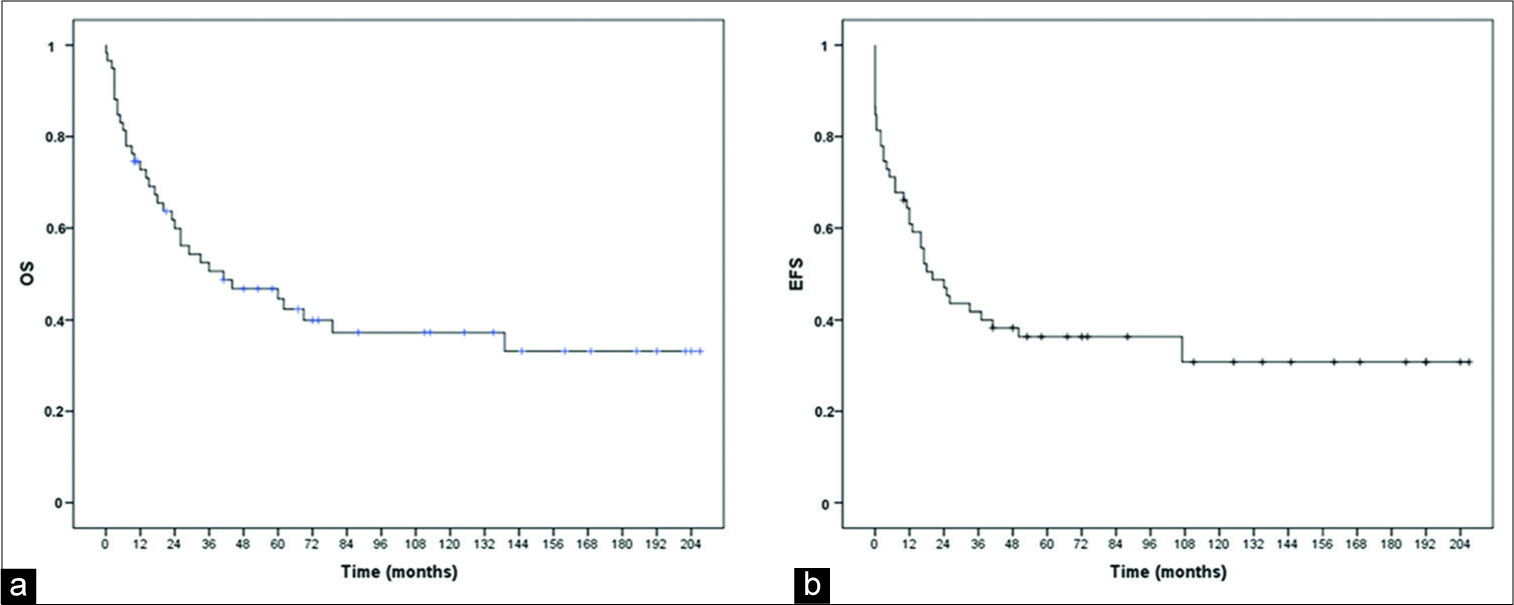
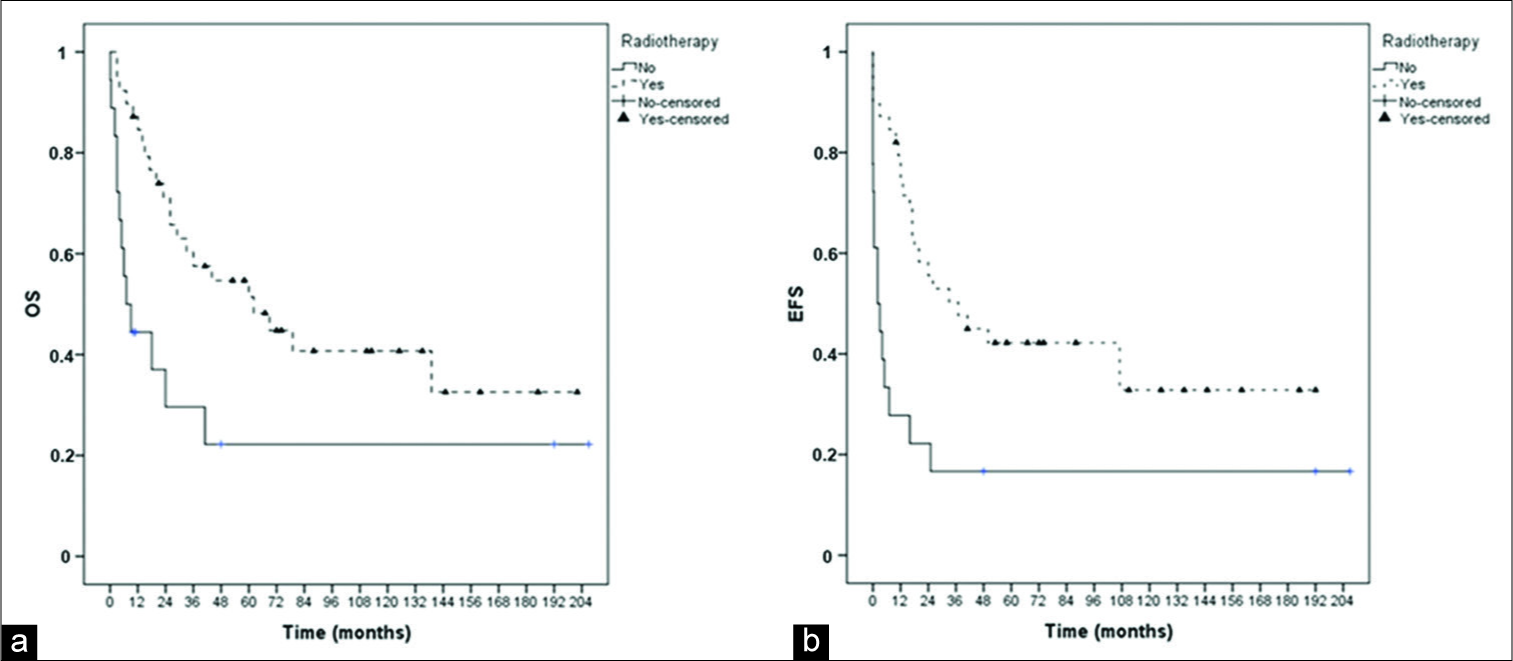
The impact of surgical resection, radiotherapy, and CT protocols on OS and EFS is shown in the Kaplan–Meier curves in
Of the total number of patients who underwent CT, 15 had the protocol interrupted due to treatment complications (n = 1), death due to therapy complications (n = 6), or death due to disease progression (n = 8). All patients who underwent RT completed the protocol.
Multivariable analysis of clinical risk factors
Neither age, PDSI, need of hydrocephalus definitive treatment, nor tumor relapse had a prognostic impact. Postoperative complications were statistically significant on bivariate analysis, although multivariate analysis suggested the opposite (padjusted = 0.415). Metastatic disease at diagnosis was the only variable identified in this cohort that had significance (P = 0.022, HR 2.76; IC 1.16–6.58). Histology was not assessed in this cohort due to lack of information on histological subclassification and central pathology review. Only seven tumor specimens were categorized according to the WHO histological subclassification.
DISCUSSION
During the 90s, OS in medulloblastomas improved with the inclusion of routine craniospinal radiation and CT protocols. At that time, several multicentric trials assessing treatment results on medulloblastoma began and are still in progress. The evaluation of the described OS and EFS can achieve rates as high as 90% in some trials.[
Most publications on medulloblastoma patients’ survival describe the results of clinical trials, which have stringent eligibility criteria that necessarily influence the survival data.[
In regard to epidemiological data, population-based studies are more reliable. Weil et al., analyzing the SEER data (Surveillance, Epidemiology, and End Results Program – a central cancer registry of the U.S.), identified the year of 1990 as a critical time-point. They labeled two cohorts, the historic one (1973–1989) and the contemporary one (1990–2012), and compared their 5-year OS. They observed that OS ranged from 51% to 69% among both, thus being statistically significant (P < 0.001).[
The 5-year OS and EFS rates gathered from this cohort analysis were lower than those in the current scientific literature, despite the fact the extent of resection was 76.8% in accordance with the data reported in literature.[
The average time interval between symptom onset and diagnosis in our review has a median time of 30 days, in accordance with current data. Reulecke et al. reported an interval of 24 days in a German center, while Dobrovoljac et al. reported a higher interval of 60 days.[
In contrast, our series comprises patients at high-risk stratification, including those younger than 3 years old. We also observed that among patients under CT protocols (n = 53), 15 had treatment interruption due to medical complications or death, meaning a loss of 30%. Von Hoff et al. reported that 70% of the patients under Packer CT protocol in the HIT91 cohort needed CT dose reduction due to toxicity, though all of them completed at least four cycles. Their analysis did not find any negative influence on survival rates.[
When we analyzed data according to stratification risk, the achieved rates in our cohort were more similar to other population-based studies. Fairley et al. found an OS of 54% in 5 years for children under 14 years old in the U.K.[
Focusing on children younger than 3 years old, we observed an OS rate in 5 and 10 years of 45.9%. None of them underwent radiotherapy protocols, receiving only high dose CT. Rutkowski et al., in a meta-analysis evaluating survival and prognostic factors in children under 5 years old, found OS in 8 years of 56%. Prognostic factors included the extent of resection, metastatic stage, and the presence of desmoplasia/extensive nodularity and anaplasia/large cell tumor in histology.[
In our analysis, we did not prove that younger children had a worse prognosis. On the contrary, our 5-year survival rates are similar in both groups (P = 0.92). Comparing our 5-year and 10-year OS, as well as EFS rates, especially in the youngest population, we observed minimal differences between them. Our case losses, therefore, occurred mostly before the 5-year follow- up period. As for the death in our cohort, we found that only one happened 6 years after the diagnosis. Another series found that 8 years following the initial diagnosis is a critical time point after which the odds of mortality from medulloblastoma are much lower when compared to all other-cause mortality.[
We achieved gross total resection in 76.8% of the children in our series. This number is in accordance with the current scientific literature.[
Curiously, hydrocephalus definitive treatment and postoperative complications were correlated to worse outcome in bivariate analysis. Shunt placement or endoscopic third ventriculostomy was needed in almost 50% of patients and those had a 188% greater chance of dying from the tumor. However, on multivariate analysis, it was not statistically significant for OS (P = 0.188). This might be related to the clinical presentation, but further studies are necessary.
There are several limitations to this study. It was not possible to perform both central pathological and radiological reviews due to the lack of access to the paraffin-embedded tissue blocks, as well as to the neuroaxis exams pre-MRI era. Furthermore, metastatic stage analysis was not possible due to the small number of patients in M2/M3 groups, as well as bias from false negative neuroimaging exams from the preMRI era and incomplete information from CSF sample acquisition. Finally, this study is subject to all the potential biases of a retrospective cohort.
CONCLUSION
As far as we know, this retrospective cohort is the largest one in Brazil that has evaluated medulloblastoma treatment outcome. Available information in literature is commonly derived from multicenter clinical randomized trials that include several countries, and sample sizes can reach hundreds of patients. Despite a limited sample, we were able to analyze OS and EFS rates in our institution, a typical public health system hospital in Brazil. The results that we have found are similar to population-based studies from the past two decades. Nevertheless, one should consider that working in a developing country, not rarely we face more difficulties in promoting the more appropriate treatment for medulloblastomas patients.
Similarly to other series, we found prognostic factors to be the extent of resection, the presence of metastatic disease, and posterior fossa and craniospinal irradiation. On the other hand, children younger than 3 years old were not correlated to a worse prognosis.
Finally, the study of this cohort of medulloblastoma epidemiological data provides the main features of this significant pathology in Southern Brazil, and we soon hope to be able to perform the molecular classification, which will provide the best treatment advances for our patients.
References
1. Chang CH, Housepian EM, Herbert C. An operative staging system and a megavoltage radiotherapeutic technic for cerebellar medulloblastomas. Radiology. 1969. 93: 1351-9
2. Chern JJ, Rao G, Lang FF, Winn R.editors. Medulloblastoma. Youmans Neurological Surgery. Philadelphia, PA: Elsevier Health Sciences; 2011. p.
3. Dhall G, Grodman H, Ji L, Sands S, Gardner S, Dunkel IJ. Outcome of children less than three years old at diagnosis with non-metastatic medulloblastoma treated with chemotherapy on the‘‘head start’’ I and II protocols. Pediatr Blood Cancer. 2008. 50: 1169-75
4. Dobrovoljac M, Hengartner H, Boltshauser E, Grotzer MA. Delay in the diagnosis of paediatric brain tumours. Eur J Pediatr. 2002. 161: 663-7
5. Ellison DW, Kocak M, Dalton J, Megahed H, Lusher ME, Ryan SL. Definition of disease-risk stratification groups in childhood medulloblastoma using combined clinical, pathologic, and molecular variables. J Clin Oncol. 2011. 29: 1400-7
6. Fairley L, Picton SV, McNally RJ, Bailey S, McCabe MG, Feltbower RG. Incidence and survival of children and young people with central nervous system embryonal tumours in the North of England, 1990-2013. Eur J Cancer. 2016. 61: 36-43
7. Gajjar A, Chintagumpala M, Ashley D, Kellie S, Kun LE, Merchant TE. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St jude medulloblastoma-96): Long-term results from a prospective, multicentre trial. Lancet Oncol. 2006. 7: 813-20
8. Johnston DL, Keene D, Kostova M, Strother D, Lafay-Cousin L, Fryer C. Incidence of medulloblastoma in Canadian children. J Neurooncol. 2014. 120: 575-9
9. Kortmann RD, Kühl J, Timmermann B, Mittler U, Urban C, Budach V. Postoperative neoadjuvant chemotherapy before radiotherapy as compared to immediate radiotherapy followed by maintenance chemotherapy in the treatment of medulloblastoma in childhood: Results of the German prospective randomized trial HIT ‘91. Int J Radiat Oncol Biol Phys. 2000. 46: 269-79
10. Kukal K, Dobrovoljac M, Boltshauser E, Ammann RA, Grotzer MA. Does diagnostic delay result in decreased survival in paediatric brain tumours. ? Eur J Pediatr. 2009. 168: 303-10
11. Lannering B, Rutkowski S, Doz F, Pizer B, Gustafsson G, Navajas A. Hyperfractionated versus conventional radiotherapy followed by chemotherapy in standard-risk medulloblastoma: Results from the randomized multicenter HIT-SIOP PNET 4 trial. J Clin Oncol. 2012. 30: 3187-93
12. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK.editorsWHO Classification of Tumors of the Central Nervous System. Lyon: International Agency for Research on Cancer; 2007. p.
13. Massimino M, Biassoni V, Gandola L, Garrè ML, Gatta G, Giangaspero F. Childhood medulloblastoma. Crit Rev Oncol Hematol. 2016. 105: 35-51
14. McKean-Cowdin R, Razavi P, Barrington-Trimis J, Baldwin RT, Asgharzadeh S, Cockburn M. Trends in childhood brain tumor incidence, 1973-2009. J Neurooncol. 2013. 115: 153-60
15. Packer RJ, Cogen P, Vezina G, Rorke LB. Medulloblastoma: Clinical and biologic aspects. Neuro Oncol. 1999. 1: 232-50
16. Packer RJ, Macdonald T, Vezina G, Grisold W, Soffietti R.editors. Medulloblastoma and primitive neuroectodermal tumors. Handbook of Clinical Neurology. Philadelphia, PA: Elsevier; 2012. p. 529-48
17. Packer RJ, Sutton LN, Goldwein JW, Perilongo G, Bunin G, Ryan J. Improved survival with the use of adjuvant chemotherapy in the treatment of medulloblastoma. J Neurosurg. 1991. 74: 433-40
18. Packer RJ, Zhou T, Holmes E, Vezina G, Gajjar A. Survival and secondary tumors in children with medulloblastoma receiving radiotherapy and adjuvant chemotherapy: Results of children’s oncology group trial A9961. Neuro Oncol. 2013. 15: 97-103
19. Pinho RS, Andreoni S, Silva NS, Cappellano AM, Masruha MR, Cavalheiro S. Pediatric central nervous system tumors: A single-center experience from 1989 to 2009. J Pediatr Hematol Oncol. 2011. 33: 605-9
20. Reulecke BC, Erker CG, Fiedler BJ, Niederstadt TU, Kurlemann G. Brain tumors in children: Initial symptoms and their influence on the time span between symptom onset and diagnosis. J Child Neurol. 2008. 23: 178-83
21. Rutkowski S, Bode U, Deinlein F, Ottensmeier H, Warmuth-Metz M, Soerensen N. Treatment of early childhood medulloblastoma by postoperative chemotherapy alone. N Engl J Med. 2005. 352: 978-86
22. Smoll NR. Relative survival of childhood and adult medulloblastomas and primitive neuroectodermal tumors (PNETs). Cancer. 2012. 118: 1313-22
23. Taylor RE, Bailey CC, Robinson K, Weston CL, Ellison D, Ironside J. Results of a randomized study of preradiation chemotherapy versus radiotherapy alone for nonmetastatic medulloblastoma: The international society of paediatric oncology/United Kingdom children’s cancer study group PNET-3 study. J Clin Oncol. 2003. 21: 1581-91
24. von Hoff K, Hinkes B, Gerber NU, Deinlein F, Mittler U, Urban C. Long-term outcome and clinical prognostic factors in children with medulloblastoma treated in the prospective randomised multicentre trial HIT’91. Eur J Cancer. 2009. 45: 1209-17
25. Weil AG, Wang AC, Westwick HJ, Ibrahim GM, Ariani RT, Crevier L. Survival in pediatric medulloblastoma: A population-based observational study to improve prognostication. J Neurooncol. 2017. 132: 99-107
26. Zeltzer PM, Boyett JM, Finlay JL, Albright AL, Rorke LB, Milstein JM. Metastasis stage, adjuvant treatment, and residual tumor are prognostic factors for medulloblastoma in children: Conclusions from the children’s cancer group 921 randomized phase III study. J Clin Oncol. 1999. 17: 832-45