- Department of Neurosurgery, Montefiore Medical Center, Bronx, New York, United States.
- Department of Neurosurgery, The Albert Einstein College of Medicine, Bronx, New York, United States.
- Department of Radiation Oncology, Montefiore Medical Center, Bronx, New York, United States.
Correspondence Address:
Rose Fluss
Department of Neurosurgery, Montefiore Medical Center, Bronx, New York, United States.
DOI:10.25259/SNI_291_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Andrew J. Kobets1, Reid Backus1, Rose Fluss2, Alan Lee3, Patrick A. Lasala1. Evaluating the natural growth rate of metastatic cancer to the brain. 21-Aug-2020;11:254
How to cite this URL: Andrew J. Kobets1, Reid Backus1, Rose Fluss2, Alan Lee3, Patrick A. Lasala1. Evaluating the natural growth rate of metastatic cancer to the brain. 21-Aug-2020;11:254. Available from: https://surgicalneurologyint.com/surgicalint-articles/10221/
Abstract
Background: Brain metastases are becoming increasingly more prevalent as cancer patients survive longer with both improved local and systemic therapy. Little is known, however, of the natural growth rates of brain metastases. This investigation aims to ascertain this growth rate of these lesions before the initiation of any CNS- directed therapy.
Methods: A total of 700 patients were screened, identifying 18 cancer patients (13 breast and 5 lung) with 29 brain metastases that were serially imaged from 2011 to 2017 before treatment for their intracranial metastases. Growth rates were measured by contouring lesions serially across at least two MRI studies in iPlan software by independent raters. These values were then compared between primary (breast and lung) cancer cohorts.
Results: The mean age at diagnosis was 53 and 95% were female. The interval between primary cancer diagnosis and brain metastases was 4.6 years and 1.2 years in the breast and lung cancer groups, respectively. Of the breast and lung cancer patients, 23% and 40% were deceased, with respective 5.08 cm3 and 2.44 cm3 initial tumor volumes. The average growth rate of lung and breast tumors was 0.018 and 0.040 cm3/day, respectively, with deceased patients having larger and faster growing tumors. Breast and lung metastases grew 2.39% and 1.14% of their total volumes daily and doubling times were 86 and 139 days, respectively.
Conclusion: This investigation provides a unique perspective into the biological growth of metastatic brain lesions. It is our hope that this study guides timing of treatment and informs both clinicians and patients of tumor growth kinetics before initiating treatment for intracranial metastases.
Keywords: Brain metastases, Breast cancer, Lung cancer, Metastatic growth, Tumor kinetics
INTRODUCTION
Over 170,000 patients develop metastatic brain tumors in the United States annually and 10–40% of the patients with malignant cancers develop brain metastases.[
Despite the myriad reports that document growth rates of brain metastases after systemic chemotherapy and local radiotherapy, few have studied natural growth rates before the initiation of therapy.[
MATERIALS AND METHODS
The databases of both the Departments of Neurosurgery and Radiation Oncology at Montefiore Medical Center were mined to identify all patients with brain metastases from 2011 to 2017. From these patients, those who received at least two MRI studies before the initiation of radiation therapy or intracranial surgery were included in this analysis. All additional follow-up was also performed during this time period. Any subsequent imaging after intracranial treatment was not used, but follow-up was continued to determine mortality rates among these patients, even after treatment. Eighteen patients (having a total of 29 brain metastases) underwent successive MRIs before any form of intracranial treatment and were included in this study. Patient’s medical records were reviewed for demographic, oncologic, radiographic, and treatment information. Permission was granted by the Albert Einstein College of Medicine Institutional Review Board to undertake this study.
To measure the growth rate of these tumors, Phillips 3T MRIs were loaded into iPlan software (Brain Lab, Munich, Bavaria), which is normally used at our institution to plan SRS treatments [
RESULTS
Case selection and demographics
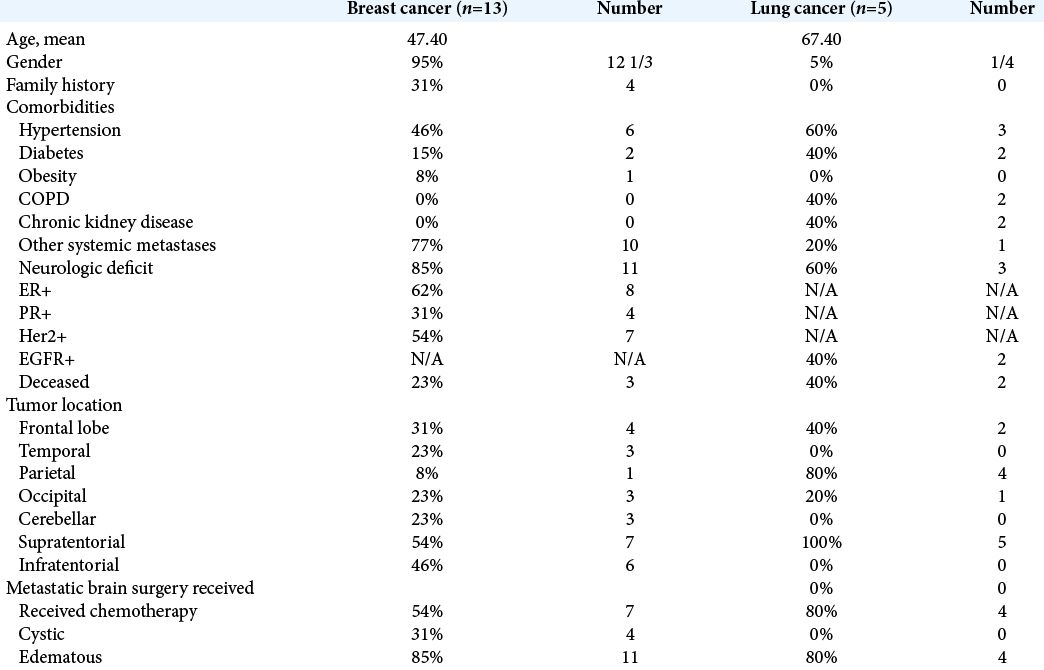
Of the 700 patients with lung and breast cancer presenting to the radiation oncology and neurosurgery services at a major New York medical center over the past 7 years, 154 were identified to have brain metastases (22%). Eighteen (having a total of 29 brain metastases) underwent successive MRIs before any form of intracranial treatment and were the few deemed eligible for inclusion in this study. Thirteen of these patients had primary breast cancer and five primary lung cancer. These patients represented 2.6% of all patients with lung and breast cancers and 12% of all patients with lung and breast cancer metastases. All but one patient was female (95% female) and the mean age was 53 years (range 28–81 years), with breast cancer patients representing the younger cohort, with a mean age of 47 versus 67 years. The average number of comorbidities per patient was 1.72, the most common being hypertension, hypercholesterolemia, and diabetes. About 25% of breast cancer patients had a family history of cancer and none of the lung cancer cohort. About 83% of patients presented with neurological symptoms, most commonly with headache, blurry vision, and nausea and vomiting. Major neurological symptoms including seizure, altered mental status, and hemiparesis occurred in 28% of patients. These symptoms were the impetus for obtaining imaging studies, with the remainder of patients undergoing surveillance imaging studies.
Only two of the 13 breast cancer patients were triple negative receptor bearers, and all lung cancer patients had nonsmall cell lung cancer lesions, with two EGFR positive. All were adenocarcinomas. One breast cancer patient had Stage I disease, two with Stage II disease, and 10 with Stage IV disease. The lung cancer patients presented with a wide range of initial stages, T1N1M0 disease in two patients, T2N2M0 in two other patients, and one patient with T3N3M1 disease. At the time of brain metastasis diagnosis, 61% of patients (77% breast and 20% lung) had metastases at other locations within the body, with the spine and liver being the most common sites. All patients underwent disease-specific primary cancer treatment including local surgical resection radiation therapy and non-CNS penetrating chemotherapy. At the time of review, five patients were deceased, 2/5 (40%) in the lung cancer group, and 3/13 (23%) of the breast cancer group [
Tumor characteristics
The mean interval from primary lung cancer diagnosis to brain metastasis diagnosis was 1.17 years and 4.64 years in the breast cancer group. Patients had an average 1.6 lesions, with a range of 1–3 lesions in both primary cancer groups. The majority of which were supratentorial, with only 5/29 (17%) located in the posterior fossa, predominantly in the cerebellar hemispheres. The supratentorial lesions were evenly distributed throughout the cerebral lobes, with only two lesions being dural based. Four lesions were cystic (22%) and the majority (83%) had significant surrounding edema. The overwhelming majority of patients only had two serial MRIs before undergoing treatment or electing for hospice care.
Tumor size
The measurements of tumor volumes demonstrated inter- rater reliability of greater than 95% and this method improved on previously extrapolated measurements based on pixel recognition used in other studies in the past.[
The second MRI time point was on average 54.05 days after the first, with the scan interval significantly longer for the breast cancer cohort, 69.7 days, compared to 13.4 days for the lung cancer group. The mean scan interval was actually shorter for the deceased cohort compared to the living cohort, 35.4 and 61.23 days, respectively. The mean volumes at time point 2 were 2.61 cm3 and 8.27 cm3 in the lung and breast cohorts, respectively, and 6.26 cm3 and 8.93 cm3 in the living and deceased cohorts, respectively.
Tumor growth rate
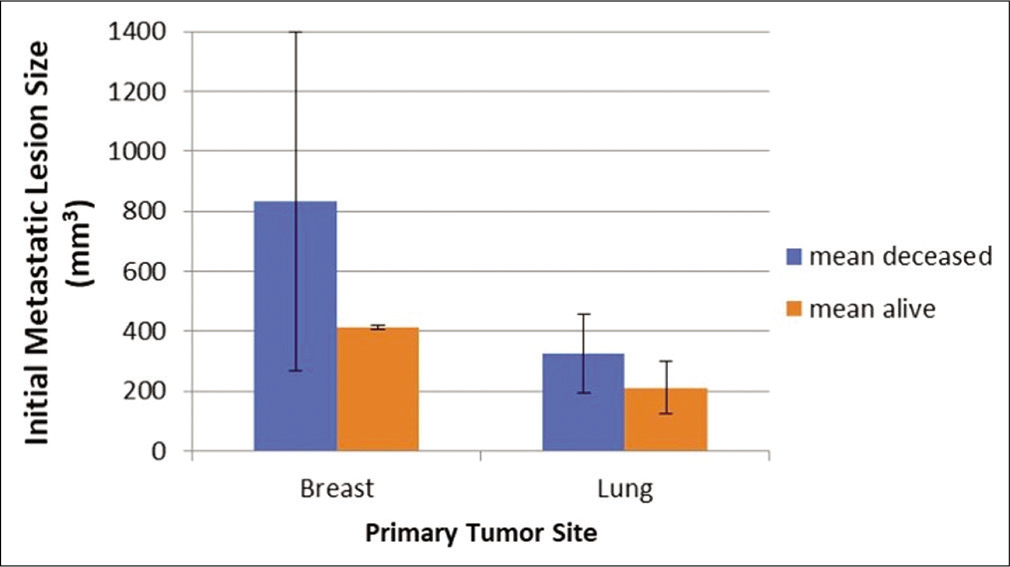
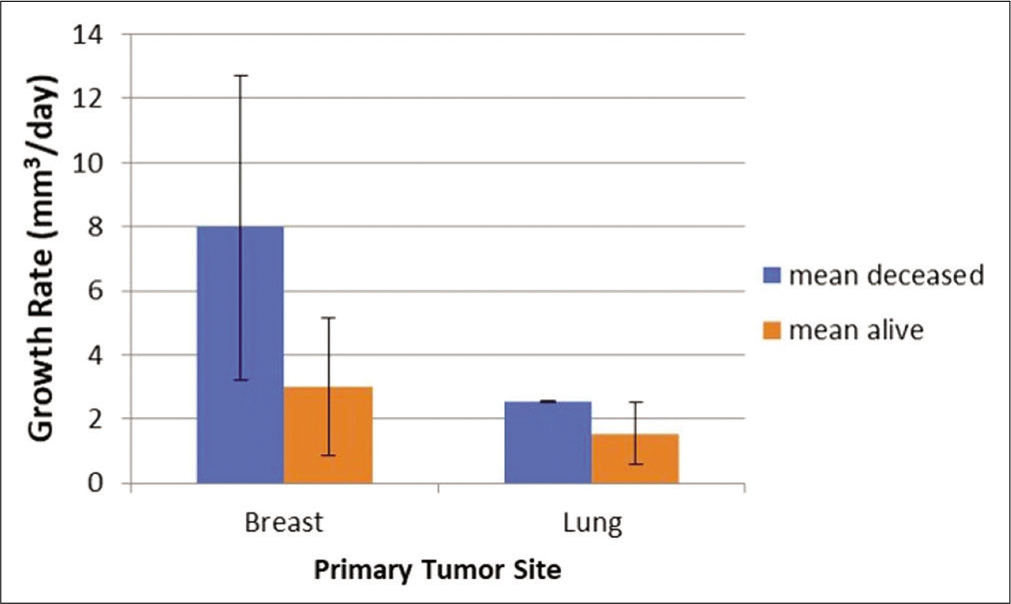
The average growth velocity for all lesions was 0.034 cm3/d (34 mm3/d) and was significantly faster in the breast cancer cohort. The mean growth rate for lung cancer metastases was 0.018 cm3/d compared to 0.04 cm3/d in the breast cancer group (P = 0.38) [
The majority of patients were fortunate enough to be treated after this initial delay. All but three patients underwent radiation therapy and all lung cancer patients received radiation therapy exclusively. Only five patients underwent surgical resection of their metastases, primarily the larger breast cancer lesions. Of the 15 patients who were radiated, three underwent whole brain radiation and the remaining 12 received stereotactic radiosurgery to their lesions.
Cystic lesions
The initial size of the cystic lesions was significantly larger than the noncystic lesions, 20.45 cm3 compared to 1.96 cm3. Cystic lesions also expectedly grew faster at a rate of 0.2 cm3/d compared to 0.026 cm3/d in the noncystic growth. Finally, 40% of the deceased patients had cystic lesions, compared to only 20% in the surviving patients. All cystic lesions, as noted, were in the breast cancer cohort, and of the two patients with triple negative receptor metastases, one patient had two predominantly cystic lesion and the other did not have any.
Triple negative breast cancer lesions
Of the 22 breast cancer lesions, four were in two patients with confirmed triple negative receptor tumors. The four triple negative breast cancer tumors demonstrated smaller than average sizes at the first time point, 0.89 cm3, yet some of the faster growing lesions of the cohort with growth velocities of 0.05 cm3/d on average.
Reasons for delaying treatment
A number of reasons were identified to better understand why patients would delay their treatments. A few patients simply decided to undergo palliation with hospice care instead of aggressive treatment and may have delayed their decision-making while a repeat image was obtained. A couple patients, despite this diagnosis of metastases, were simply lost to follow up, albeit for a short period of time, only to return to clinic to have a repeat MRI performed. One patient refused surgery and treatment, only to return 1 month later to decide on a treatment modality. It is suspected that some patients may have had issues with scheduling treatment appointments, thereby delaying treatment for a short period, requiring reimaging. Two patients, while waiting for treatment developed new neurological symptoms, including a seizure and altered mental status, which resulted in repeat imaging before planned therapeutic management.
DISCUSSION
Of 20–50% of women who develop breast cancer metastases, 80% were reported in the past to die in the 1st year after diagnosis.[
The overall expectation for survival is 1–2 months for untreated metastatic patients and 4–6 months for timely treated patients.[
Patient and tumor characteristics
It is rare that a patient will be diagnosed with a new brain metastasis and not undergo treatment shortly thereafter. Treatment should begin right after diagnosis, and the purpose of this study was to demonstrate the risk of delaying treatment. In our large cohort of 700 patients with metastases, serial imaging in untreated patients occurred only a handful of times. From these rare cases, we studied the uninhibited growths of these tumors.
As expected in a cohort of predominantly breast and lung cancer patients, most were women, in their 4th and 5th decades of life, similar to demographics found in the literature.[
This study demonstrated the mean interval from primary lung cancer diagnosis to brain metastasis was 1.17 years and 4.64 years in the breast cancer group. This is slightly longer than the average time of diagnosis of breast cancer to brain metastasis previously reported of 34 months (2.83 years). This value is unfortunately not standardized and usually reliant on clinical suspicion alone.[
Tumor size and growth
In this study, we demonstrated that lung cancer metastases grew at the same rate as a previously demonstrated in an independent prospective study.[
Natural growth may be vitally important to determine prognostic factors for the overall survival of patients, however, the strict correlation between tumor size and survival is unclear and is likely a multifaceted process in which many systemic factors may play a role.[
The positive effect of diagnosing metastases early is demonstrated in Park et al. Patients that underwent early pretreatment MRIs had a significantly higher 5-year survival rate, compared to those who did not undergo pretreatment MRIs.[
The natural growth rate of nonsmall cell lung cancer found by Yoo et al. which demonstrated the feasibility of the approach taken in this study was 0.01 cm3/day, similar to the 0.018 cm3/day rate obtained in the present cohort of lung cancer patients.[
There was a notable difference in the growth rates of the breast and lung cancer patients. Therefore, biology may play a major role in tumor growth and patient survival. However, the deceased patients did have greater than twice as fast growth of tumors when combining both groups, suggesting that tumor growth in itself, despite tumor type, was in fact relevant to survival. These data suggest that prognosis is dependent on several potentially independent factors.
The average initial lesion size in the Yoo study was 0.04 cm3 compared to 2.44 cm3 in this study, with Yoo’s cohort being previously treated. Despite the smaller tumor size, the velocities between our studies were essentially equal. Therefore, while their study may have imaged these metastases earlier or after initial treatment response, it is reassuring that the tumors appear to maintain the same growth rate throughout their existence. In addition, based on the pooled data between these studies, it is suggested that the growth rate may be linear, however, a stepwise or even logarithmic growth rate may also still be possible. Few studies have addressed this question in the literature however in a review of 22 studies involving 675 patients, the data demonstrated that growth rates of primary intracranial tumors were linear.[
An interesting finding in our study was that each day, breast cancer tumors grew 2.39% of the initially identified tumor size, and the lung cancers, 1.15% of their initial sizes. This also demonstrated that tumor doubling time was 86 days for breast cancers and 139 days for lung cancers. This was consistent with other studies that showed tumor doubling time of under 100 days for up to 1/3 of lung cancers and 40% double time between 100 and 400 days.[
Cystic tumors
Cystic tumors in this study were larger and grew faster than noncystic lesions. This can certainly be explained by the addition of the cystic cavity in the overall size of the lesions and the likelihood that cystic fluid production occurs at a faster rate than growth of solid tumor mass. Interestingly, 50% of the deceased patients had cystic lesions, compared to 20% mortality in the noncystic lesions. Mortality may have been directly linked to tumor size, but also tumor biology. Even without the consideration of these cystic lesions in the breast cancer group, the breast tumors grew faster and had larger initial sizes than the lung cancer group.
Triple negative breast cancer
Triple negative tumors have worse prognostic outcomes than other breast cancer types with a mean survival between 3.4 and 4.9 months after diagnosis.[
Treatment delays
Reasons for treatment delays varied and included election to not be treated, decision to undergo hospice care, failure of the patient to follow-up, scheduling delays, and finally, patients needing to take time to make a decision on treatment choice. Some of these delays were preventable by having doctors thoroughly explain the need for prompt treatment due to tumor growth to their patients. All attempts should have been made to contact patients lost to follow up, and treatment initiated as soon as possible. Of course, all of these issues and patient desires must be balanced in a system that is equitable to all, for both the individual and for the society.
Limitations
The limited number of patients, due to the criteria of the study, weakens the statistical strength of the data. Larger cohorts of data may in the future demonstrate significance in a number of measures in our study that were approaching significance. While the lesions had to be manually contoured, using multiple blinded raters demonstrated a greater than 95% inter-rater reliability, error in true volumetric measurements may have occurred. Older, less clear images, and tumor edema may have contributed to imprecise contouring of lesions. It is possible that patients with metastases who refused all treatments and never presented to either the radiation oncology nor neurosurgery services would have been missed in this analysis. The cystic growth of lesions could have skewed the breast data; however, this is legitimate and significant volumetric information, contributing to intracranial mass effect. Breast lesions were still larger and faster growing despite these lesions. The patient charts may have been incomplete and there could have been additional deceased patients in our cohort, although each patient had notes written within 3 months of this report. Despite these limitations, the authors feel that the data are meaningful and may inform patient care in the future.
CONCLUSION
The data presented in this report demonstrate that metastatic tumors to the brain grow at substantial rates per day, calling into action patients and clinicians to not delay treatment for these lesions, whenever possible. These data have previous not been reported in the literature and have elucidated reason at least in our cohort as to why delays have occurred in the past. Further studies should evaluate tumor growth in more than 2 serial pretreatment scans to confirm whether growth of these lesions is linear, exponential, or stepwise, as well as study much larger cohorts of patients to determine the statistical significance of growth rates between patients and tumor types. In addition, other types of metastases may be evaluated and prospective trials may be conducted to evaluate whether outcomes improve in patients who are hyperacutely treated, compared to those who undergo standard treatment regimens.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Amsbaugh M, Pan J, Yusuf MB, Dragun A, Dunlap N, Guan T. Dose-volume response relationship for brain metastases treated with frameless single-fraction linear accelerator-based stereotactic radiosurgery. Cureus. 2016. 8: e587
2. Chang EL. Diagnosis and management of central nervous system metastases from breast cancer. Oncologist. 2003. 8: 398-410
3. Dawood S, Lei X, Litton JK, Buchholz TA, Hortobagyi GN, Gonzalez-Angulo AM. Incidence of brain metastases as a first site of recurrence among women with triple receptor-negative breast cancer. Cancer. 2012. 118: 4652-9
4. Distefano A, Yap YY, Hortobagyi GN, Blumenschein GR. The natural history of breast cancer patients with brain metastases. Cancer. 1979. 44: 1913-8
5. Ebner D, Rava P, Gorovets D, Cielo D, Hepel JT. Stereotactic radiosurgery for large brain metastases. J Clin Neurosci. 2015. 22: 1650-4
6. Feigl GC, Horstmann GA. Volumetric follow up of brain metastases: A useful method to evaluate treatment outcome and predict survival after Gamma Knife surgery?. J Neurosurg. 2006. 105: 91-8
7. Hambrecht A, Jandial R, Neman J. Emerging role of brain metastases in the prognosis of breast cancer patients. Tumors Cent Nerv Syst. 2011. 4: 349-63
8. Hines SL, Vallow LA, Tan WW, Mcneil RB, Perez EA, Jain A. Clinical outcomes after a diagnosis of brain metastases in patients with estrogen-and/or human epidermal growth factor receptor 2-positive versus triple-negative breast cancer. Ann Oncol. 2008. 19: 1561-5
9. Iyer A, Harrison G, Kano H, Weiner GM, Luther N, Niranjan A. Volumetric response to radiosurgery for brain metastasis varies by cell of origin. J Neurosurg. 2014. 121: 564-9
10. Jaboin JJ, Ferraro DJ, DeWees TA, Rich KM, Chicoine MR, Dowling JL. Survival following gamma knife radiosurgery for brain metastasis from breast cancer. Radiat Oncol. 2013. 8: 131
11. Leone JP, Lee AV, Brufsky AM. Prognostic factors and survival of patients with brain metastasis from breast cancer who underwent craniotomy. Cancer Med. 2015. 4: 989-94
12. Lindell RM, Hartman TE, Swensen SJ, Jett JR, Midthun DE, Tazelaar HD. Five-year lung cancer screening experience: CT appearance, growth rate, location, and histologic features of 61 lung cancers. Radiology. 2007. 242: 555-62
13. Niwi-242 A, Murawska M, Pogoda K. Breast cancer subtypes and response to systemic treatment after whole-brain radiotherapy in patients with brain metastases. Cancer. 2010. 116: 4238-47
14. Ogura M, Mitsumori M, Okumura S, Yamauchi C, Kawamura S, Oya N. Radiation therapy for brain metastases from breast cancer. Breast Cancer. 2003. 10: 349-55
15. Olivero WC, Lister JR, Elwood PW. The natural history and growth rate of asymptomatic meningiomas: A review of 60 patients. J Neurosurg. 1995. 83: 222-4
16. Park HY, Kim YH, Kim H, Koh WJ, Suh GY, Chung MP. Routine screening by brain magnetic resonance imaging decreased the brain metastasis rate following surgery for lung adenocarcinoma. Lung Cancer. 2007. 58: 68-72
17. Patchell RA. The management of brain metastases. Cancer Treat Rev. 2003. 29: 533-40
18. Rostami R, Mittal S, Rostami P, Tavassoli F, Jabbari B. Brain metastasis in breast cancer: A comprehensive literature review. J Neurooncol. 2016. 127: 407-14
19. Serres S, Soto MS, Hamilton A, McAteer MA, Carbonell WS, Robson MD. Molecular MRI enables early and sensitive detection of brain metastases. Proc Natl Acad Sci U S A. 2012. 109: 6674-9
20. Shi WM, Wildrick DM, Sawaya R. Volumetric measurement of brain tumors from MR imaging. J Neurooncol. 1998. 37: 87-93
21. Steele JD, Buell P. Asymptomatic solitary pulmonary nodules. Host survival, tumor size, and growth rate. J Thorac Cardiovasc Surg. 1973. 65: 140-51
22. Sul J, Posner JB.editors. Brain metastases: Epidemiology and pathophysiology. Brain Metastases Cancer Treatment and Research. Boston, MA: Springer; 2007. p. 1-21
23. Weil RJ, Palmieri DC, Bronder JL, Stark AM, Steeg PS. Breast cancer metastasis to the central nervous system. Am J Pathol. 2005. 167: 913-20
24. Yoo H, Jung E, Nam BH, Shin SH, Gwak HS, Kim MS. Growth rate of newly developed metastatic brain tumors after thoracotomy in patients with non-small cell lung cancer. Lung Cancer. 2011. 71: 205-8
25. Yoo H, Nam BH, Yang HS, Shin SH, Lee JS, Lee SH. Growth rates of metastatic brain tumors in nonsmall cell lung cancer. Cancer. 2008. 113: 1043-7