- Department of Neurosurgery, University of California, Irvine Medical Center, Orange, California,
- Department of Biomedical Engineering, University of California, Irvine School of Medicine, Irvine.
Correspondence Address:
Jefferson Chen
Department of Neurosurgery, University of California, Irvine Medical Center, Orange, California,
DOI:10.25259/SNI_324_2019
Copyright: © 2019 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Jordan Xu, Cassie Poole, Ronald Sahyouni, Jefferson Chen. Noninvasive thermal evaluation for shunt failure in the emergency room. 27-Dec-2019;10:254
How to cite this URL: Jordan Xu, Cassie Poole, Ronald Sahyouni, Jefferson Chen. Noninvasive thermal evaluation for shunt failure in the emergency room. 27-Dec-2019;10:254. Available from: https://surgicalneurologyint.com/surgicalint-articles/9824/
Abstract
Background: Ventriculoperitoneal shunts (VPSs) have been the mainstay of treating hydrocephalus since the 1950s. However, shunts have a reported complication rate reaching nearly 50%. Devices have been developed that utilize noninvasive thermal transcutaneous diffusion technology. These shunt evaluation devices measure temperature gradients to detect shunt cerebrospinal fluid flow. We assessed the utility using a thermal diffusion technique to work up shunt failure in the emergency room (ER).
Methods: This was a retrospective case series at a single medical center ER. We included consecutive patients with possible VPS malfunction who were evaluated with a thermal sensor. The time period of data collection included September 2015–April 2018.
Results: Eight patients were reviewed, including four males and four females. The average age of reviewed patients was 35.1 (+/− ; 16.5). In our case series, three patients had adequate shunt flow as assessed by the shunt evaluation device, and the decision was made to discharge the patient and follow-up in clinic. In two patients, the flow was diminished, but due to other reassuring signs, the patients were still discharged with follow-up. Two patients were noted to have poor flow and were admitted for shunt revision.
Conclusion: It is important to determine whether a malfunction is present and whether an intervention is necessary for patients who present to the emergency department with possible symptoms of shunt failure. A thermal sensor is a fast and noninvasive tool in the evaluation of shunt flow and helps determine whether it is safe to send a patient home or intervene appropriately.
Keywords: Hydrocephalus, Shunt failure, Thermal sensor, Thermosensitive, Ventriculoperitoneal shunt
INTRODUCTION
Ventriculoperitoneal shunts (VPSs) have been the mainstay of treating hydrocephalus since the 1950s and have become one of the most frequently performed neurosurgical procedures.[
The diagnosis of shunt malfunction can be difficult even for experienced clinicians. Classical symptoms of shunt malfunction are nonspecific and include headache, lethargy, nausea, and vomiting.[
Cutaneous thermal diffusion techniques are promising noninvasive and quick techniques to assess CSF flow in a shunt. The ShuntCheck (Neuro Diagnostic Devices, Inc., Trevose, Pennsylvania) device uses temperature gradients to detect and quantify shunt CSF flow. The previous studies have shown that the device reliably determines CSF flow, confirmed by flow detection in the OR, with good sensitivity and specificity.[
METHODS
This was a retrospective review at a single medical center ER with the Institutional Review Board approval. Written informed consent was waived for this study given a retrospective analysis of de-identified data. We included consecutive patients with the question of VPS malfunction who were evaluated with the thermal sensor device. The time period of data collection included September 2015–April 2018.
ShuntCheck procedure
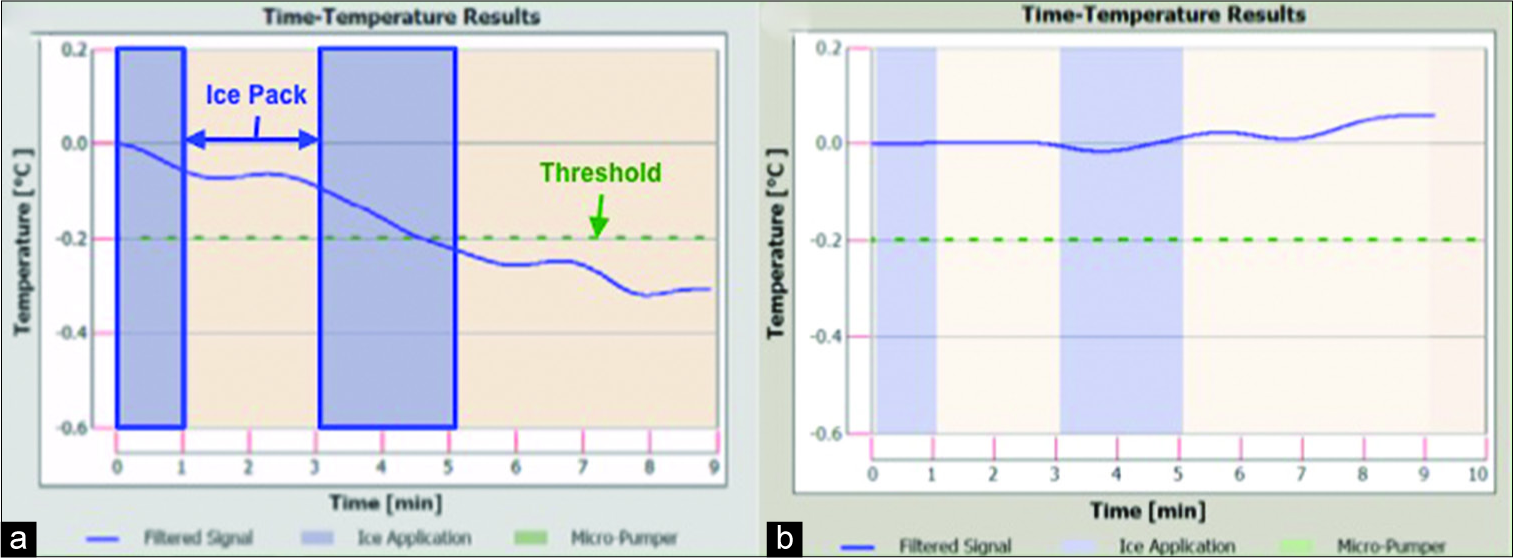
Patients are positioned either flat, at 45°, or 90°. The ShuntCheck program provides step-by-step guidance and instruction on the use of their system. The shunt tubing was palpated as it crosses the clavicle, and a thermal sensor device is placed directly over the shunt where the catheter crosses the clavicle in line with the direction of tubing. An ice pack was placed to cool the skin and underlying catheter “upstream” of the thermal detector per protocol. If CSF is flowing through the shunt, the cooled fluid will preferentially cool the region of skin above the shunt tubing and this differential will propagate “downstream” and be detected by the thermal detector. The program detects CSF flow measuring the difference in cooling of the skin above the shunt tubing relative to the flanking skin. If the device detects a characteristic downstream transcutaneous temperature dip, the computer reports: “Flow confirmed.” A quantitative value based on the temperature curve is also reported as the natural flow amplitude (NFA). If no temperature dip is detected, the unit reports: “Flow not confirmed.” A real-time temperature graph is produced [
Figure 1:
(a) Sample ShuntCheck result demonstrating a temperature gradient indicative of shunt flow; (b) sample ShuntCheck result with no temperature change suggestive of no flow; blue shaded bars represent periods of ice application; green dotted line represents threshold of temperature drop to determine adequate flow.
RESULTS
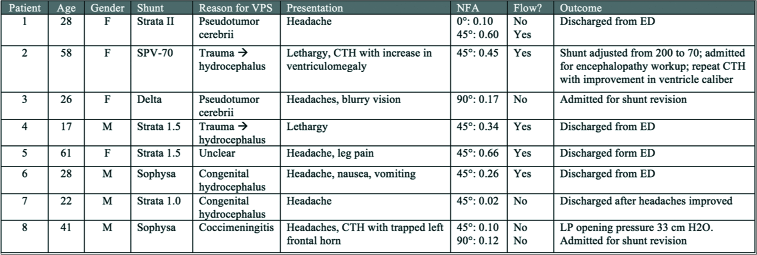
Eight patients were reviewed, including four males and four females. The average age of reviewed patients was 35.1 (±16.5) years old. The leading presenting symptom was headache, occurring in six of the eight patients. Other symptoms included lethargy, nausea, vomiting, and change in mental status. VPS devices included Medtronic Strata, Medtronic Delta, and Sophysa Polaris.
Five (62.5%) of the patients were discharged home after thermal sensor showed good flow (patients 1, 4, 5, 6, and 7). Three (37.5%) patients required intervention. One patient shunt valve setting was adjusted (patient 2) based on imaging findings showing increased ventricle caliber. Two other patients were admitted for shunt revisions based on a diagnosis of shunt failure (patients 3 and 8). In both of these patients, the thermal sensor revealed inadequate shunt flow.
Example 1 (patient 3)
A 26-year-old female with a history of idiopathic intracranial hypertension and VPS (Medtronic Delta) presented with headaches and blurry vision. Her shunt was placed 1 month before presentation at an outside hospital. CT head imaging revealed slit ventricles. LP revealed an opening pressure of 33 cm H2O. Ophthalmologic examination showed evidence of bilateral papilledema. Thermal sensor at 90° showed slow flow (NFA: 0.17). Given high suspicion of shunt failure, the patient was admitted for shunt revision. At the time of surgery, the proximal catheter was noted to have obstruction of flow and appeared adherent to the choroid. The proximal catheter was replaced along with a new Medtronic Strata valve. Follow-up appointment revealed the resolution of papilledema although headache persisted.
Example 2 (patient 6)
A 28-year-old male from Germany with a history of epilepsy and hydrocephalus since infancy secondary to traumatic brain injury, with the first VPS at 5 years of age and extensive subsequent revisions presented with headache, nausea, and vomiting. The patient had lived in Europe until 3 weeks before presentation. The most recent revision was 1 month before presentation. CT imaging showed normal ventricle caliber and shunt series did not show any discontinuity or kinking. Thermal sensor revealed good flow at 45° (NFA: 0.26). It was concluded that the shunt was functioning. Nausea and vomiting had resolved without intervention. Headaches persisted although the patient was agreeable to outpatient neurology follow-up for further headache management. The patient was discharged with instruction to follow up with both neurology and his neurosurgeon for further management of shunt.
For the patients that were discharged home, we reviewed at least 3 months of follow-up data after initial presentation. The previously discussed patient 6 had no follow-up data as the patient returned to Germany to see his original neurosurgeon. Of the remaining four patients, none required intervention in the subsequent 3 months. Patient 1 presented to the clinic 1 month later and continued to have headaches suggestive of overdrainage, so the Medtronic Strata shunt was dialed from setting 1.0 to 1.5. However, on follow-up 2 months later, this was more uncomfortable to the patient and the shunt setting was returned to 1.0. Patient 4 was seen in the clinic 2 months after the initial visit and had the Strata valve dialed down from 2.0 to 1.5. The patient had reported some improvement at another visit 2 months later. Patient 5 was seen in the clinic 4 months after presentation and noted to be doing well. Patient 7 was seen 2 and 6 months after presentation and noted to be doing well at each visit.
DISCUSSION
We demonstrate that thermal sensor is a clinically useful tool in the initial management and assessment of patients presenting with possible VPS failure. It can be used as a screening tool to either rule out obstruction or prompt further advanced testing. Clinical symptoms suggestive of VPS failure include headache, vomiting, decreased mental status, and fever. The majority of the patients included in this review had reported headaches, and the remaining two presented with a decreased level of consciousness. These symptoms are often vague and have a wide differential in etiology. Conversely, some patients with shunt failure present with none of these symptoms. There are potentially severe consequences of missing shunt failure, and it is important for the ER provider and/or neurosurgeon to make a careful and timely evaluation.
The routine workup for shunt failure often involves neuroimaging. CT and shunt series X-ray are the most common type of imaging obtained in the initial work-up. Imaging findings alone, however, are not indicative of shunt failure.[
Tapping the shunt reservoir is one objective measurement that can provide opening pressures and direct CSF analysis.[
The thermal sensor tool is useful in distinguishing whether there is flow within the shunt system and aid in the diagnosis of shunt failure. The method of assessing flow through temperature fluctuations has been around for several decades.[
All of the patients reviewed in our study portray scenarios, in which the patient is rapidly evaluated and a point-of-care method and proper disposition can be decided in a shorter amount of time.
A positive flow detected by a thermal sensor not only can reassure the clinician but also the patient. However, a shunt flow evaluation by itself is not a predictor of the clinical diagnosis of shunt failure, as the previous studies have shown.[
Limitations
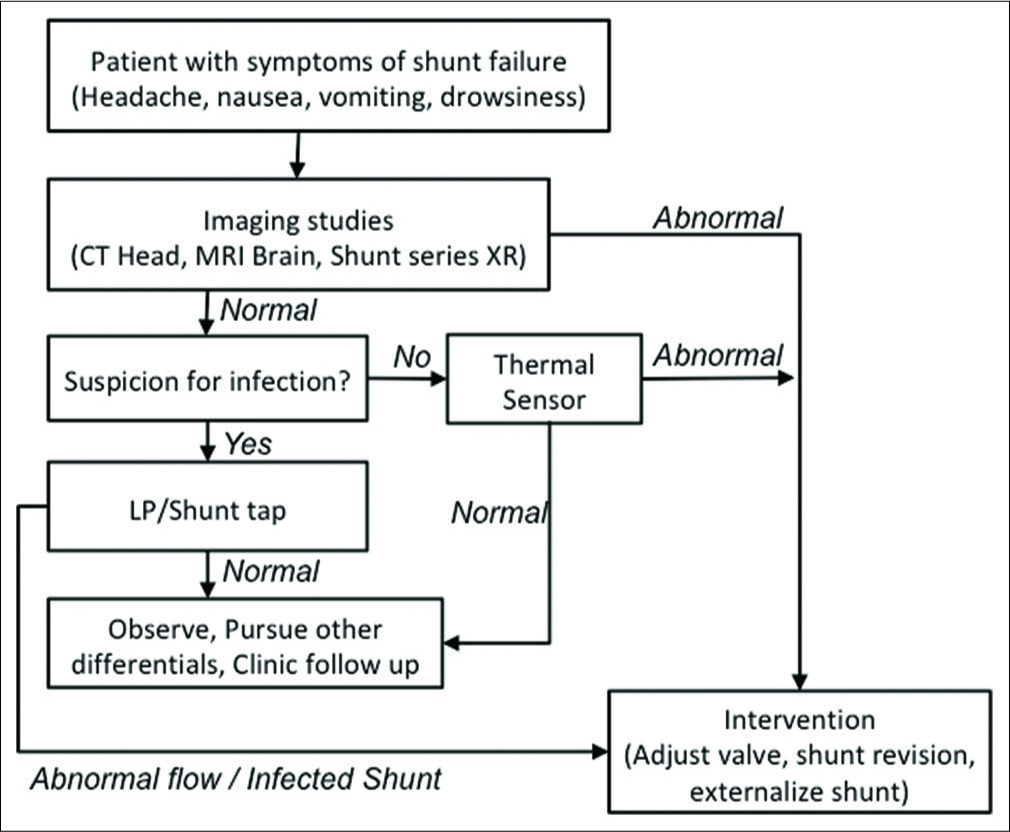
This is a small retrospective review with only eight patients, limiting the generalizability of our findings. The low number of patients is due to the fact that only a subset of patients presenting with symptoms of shunt malfunction will undergo a thermal sensor evaluation. The thermal sensor results are most useful in patients that have inconclusive presentation and imaging findings, and a further diagnostic test can be helpful. The decision tree in
CONCLUSION
It is important to determine whether shunt dysfunction is present and whether an intervention is necessary for patients who present to the ER with possible symptoms of shunt failure. A thermal sensor device is a useful, fast, portable, and non-invasive tool in the evaluation of shunt flow and can help clinicians determine whether it is safe to send a patient home or intervene appropriately.
Declaration of patient consent
Written informed consent was waived for this study given a retrospective analysis of de-identified data.
Financial support and sponsorship
Funded in part by MSTP (Medical Scientist Training Program) Grant from NIH (T32-GM08620) to RS.
Conflicts of interest
There are no conflicts of interest.
References
1. Aschoff A, Kremer P, Hashemi B, Kunze S. The scientific history of hydrocephalus and its treatment. Neurosurg Rev. 1999. 22: 67-93
2. Bondurant CP, Jimenez DF. Epidemiology of cerebrospinal fluid shunting. Pediatr Neurosurg. 1995. 23: 254-8
3. Caldarelli M, Di Rocco C, Cellini N, De Santis M. A technique for evaluation of CSF shunt patency using telethermography. Neuropediatrics. 1981. 12: 303-7
4. Chiba Y, Ishiwata Y, Suzuki N, Muramoto M, Kunimi Y. Thermosensitive determination of obstructed sites in ventriculoperitoneal shunts. J Neurosurg. 1985. 62: 363-6
5. Chiba Y, Yuda K. Thermosensitive determination of CSF shunt patency with a pair of small disc thermistors. J Neurosurg. 1980. 52: 700-4
6. Choux M, Genitori L, Lang D, Lena G. Shunt implantation: Reducing the incidence of shunt infection. J Neurosurg. 1992. 77: 875-80
7. Go KG, Melchior HJ, Lakke JP. A thermosensitive device for the evaluation of the patency of ventriculo-atrial shunts in hydrocephalus. Acta Neurochir (Wien). 1968. 19: 209-16
8. Hameed MQ, Zurakowski D, Proctor MR, Stone SSD, Warf BC, Smith ER. Noninvasive thermal evaluation of ventriculoperitoneal shunt patency and cerebrospinal fluid flow using a flow enhancing device. Neurosurgery. 2019. 85: 240-9
9. Ishiwata Y, Chiba Y, Yamashita T, Gondo G, Ide K, Kuwabara T. Thermosensitive determination of patency in lumboperitoneal shunts. Technical note. J Neurosurg. 1989. 70: 143-5
10. Iskandar BJ, McLaughlin C, Mapstone TB, Grabb PA, Oakes WJ. Pitfalls in the diagnosis of ventricular shunt dysfunction: Radiology reports and ventricular size. Pediatrics. 1998. 101: 1031-6
11. Kulkarni AV, Drake JM, Lamberti-Pasculli M. Cerebrospinal fluid shunt infection: A prospective study of risk factors. J Neurosurg. 2001. 94: 195-201
12. Kurwale NS, Agrawal D. Phase-contrast magnetic resonance imaging of intracranial shunt tube: A valuable adjunct in the diagnosis of ventriculoperitoneal shunt malfunction. Clin Neurosurg. 2011. 58: 138-42
13. Maartens NF, Aurora P, Richards PG. An unusual complication of tapping a ventriculoperitoneal shunt. Eur J Paediatr Neurol. 2000. 4: 125-9
14. Madsen JR, Abazi GS, Fleming L, Proctor M, Grondin R, Magge S. Evaluation of the ShuntCheck noninvasive thermal technique for shunt flow detection in hydrocephalic patients. Neurosurgery. 2011. 68: 198-205
15. Marlin AE, Gaskill SJ. The use of transcutaneous thermal convection analysis to assess shunt function in the pediatric population. Neurosurgery. 2012. 70: 181-3
16. Martin AJ, Drake JM, Lemaire C, Henkelman RM. Cerebrospinal fluid shunts: Flow measurements with MR imaging. Radiology. 1989. 173: 243-7
17. McNatt SA, Kim A, Hohuan D, Krieger M, McComb JG. Pediatric shunt malfunction without ventricular dilatation. Pediatr Neurosurg. 2008. 44: 128-32
18. Meier U, Mutze S. Does the ventricle size change after shunt operation of normal-pressure hydrocephalus?. Acta Neurochir Suppl. 2005. 95: 257-9
19. Merkler AE, Ch’ang J, Parker WE, Murthy SB, Kamel H. The Rate of complications after ventriculoperitoneal shunt surgery. World Neurosurg. 2017. 98: 654-8
20. Miller JP, Fulop SC, Dashti SR, Robinson S, Cohen AR. Rethinking the indications for the ventriculoperitoneal shunt tap. J Neurosurg Pediatr. 2008. 1: 435-8
21. Neff S. Measurement of flow of cerebrospinal fluid in shunts by transcutaneous thermal convection. Technical note. J Neurosurg. 2005. 103: 366-73
22. Noetzel MJ, Baker RP. Shunt fluid examination: Risks and benefits in the evaluation of shunt malfunction and infection. J Neurosurg. 1984. 61: 328-32
23. Paff M, Alexandru-Abrams D, Muhonen M, Loudon W. Ventriculoperitoneal shunt complications: A review. Interdisciplinary Neurosurgery. 2018. 13: 66-70
24. Park MK, Kim M, Park KS, Park SH, Hwang JH, Hwang SK. A retrospective analysis of ventriculoperitoneal shunt revision cases of a single institute. J Korean Neurosurg Soc. 2015. 57: 359-63
25. Reddy GK, Bollam P, Caldito G. Long-term outcomes of ventriculoperitoneal shunt surgery in patients with hydrocephalus. World Neurosurg. 2014. 81: 404-10
26. Sood S, Kim S, Ham SD, Canady AI, Greninger N. Useful components of the shunt tap test for evaluation of shunt malfunction. Childs Nerv Syst. 1993. 9: 157-61
27. Spirig JM, Frank MN, Regli L, Stieglitz LH. Shunt age-related complications in adult patients with suspected shunt dysfunction. A recommended diagnostic workup. Acta Neurochir (Wien). 2017. 159: 1421-8
28. Stein SC. Testing cerebrospinal fluid shunt function: A noninvasive technique. Neurosurgery. 1980. 6: 649-51
29. Winston KR, Lopez JA, Freeman J. CSF shunt failure with stable normal ventricular size. Pediatr Neurosurg. 2006. 42: 151-5
30. Wu Y, Green NL, Wrensch MR, Zhao S, Gupta N. Ventriculoperitoneal shunt complications in California: 1990 to 2000. Neurosurgery. 2007. 61: 557-62