- Department of Neurosurgery, Gyotoku General Hospital, Ichikawa, Chiba, Japan
Correspondence Address:
Yuhei Michiwaki, Department of Neurosurgery, Gyotoku General Hospital, Ichikawa, Chiba, Japan.
DOI:10.25259/SNI_232_2025
Copyright: © 2025 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Yuhei Michiwaki, Yusuke Takamine, Takahiro Kumagawa, Chihiro Yagi, Ryo Kajiwara, Ryo Otaki, Seiichiro Mine, Takuji Igarashi. A case of recurrent hemangioblastoma receiving blood supply from the mastoid and transosseous branches of the occipital artery. 02-May-2025;16:167
How to cite this URL: Yuhei Michiwaki, Yusuke Takamine, Takahiro Kumagawa, Chihiro Yagi, Ryo Kajiwara, Ryo Otaki, Seiichiro Mine, Takuji Igarashi. A case of recurrent hemangioblastoma receiving blood supply from the mastoid and transosseous branches of the occipital artery. 02-May-2025;16:167. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13529
Abstract
BackgroundHemangioblastomas (HBs) typically receive their blood supply from the branches of the intracranial arteries; however, meningeal branches or arteries originating from the external carotid artery are rare because of the intramedullary subpial location of the HBs. Although HB embolization is effective, it carries complication risks.
Case DescriptionA 71-year-old man with a history of incomplete HB resection consulted our hospital with headache, vertigo, and nausea. Neuroimaging revealed a recurrent HB. Angiography demonstrated that the HB was fed by mastoid branch (MB) and transosseous branches (TOBs) from the occipital artery (OA), in addition to the superior cerebellar artery (SCA) and the posterior inferior cerebellar artery (PICA). The patient underwent preoperative embolization with n-butyl-2-cyanoacrylate through the SCA and PICA branches. After embolization for TOBs, which led to feeder occlusion of the OA, the meningeal branch of the MB from the OA was revealed. We hesitated to perform embolization targeting this MB, considering the risk of potential anastomosis to the vertebral artery. Total resection through an enlarged craniectomy was conducted with minimal bleeding. Postoperative magnetic resonance imaging revealed no remnant tumor; however, infarction was observed in the area perfused by the SCA due to embolization of the SCA branches. The symptoms improved after surgery, and the patient was discharged following rehabilitation, with slight ataxia as a sequela.
ConclusionThis is a rare case of recurrent HB receiving a blood supply from the MB and TOBs from the OA. Thus, embolization for TOBs is safe and effective for recurrent HB resection.
Keywords: Embolization, Hemangioblastoma, Infarction, Mastoid branch, Transosseous branch
INTRODUCTION
Hemangioblastomas (HBs) are benign vascular tumors of the central nervous system, representing 1–2.5% of all brain tumors, and commonly arise in the posterior cranial fossa. Cerebral angiography is a standard diagnostic method for HBs that detect feeding arteries and venous drainage. HBs typically receive blood supply from the branches of the intracranial arteries, such as the superior cerebellar artery (SCA), anterior inferior cerebellar artery (AICA), and posterior inferior cerebellar artery (PICA). Blood supply to HBs from the meningeal branches or arteries from the external carotid artery (ECA) is rare owing to the intramedullary subpial location of the HBs.[
The aim of HB treatment is complete resection, which could be curative. However, complete resection of HBs is challenging because internal decompression or piecemeal resection could lead to devastating intraoperative bleeding.[
Herein, we present the rare case of a patient with recurrent HB who received blood supply from the mastoid branch (MB) and transosseous branches (TOBs) of the occipital artery (OA) and underwent surgical resection after embolization.
CASE DESCRIPTION
A 71-year-old man was referred to our department with headache, vertigo, and nausea that had persisted for several days. The patient had undergone craniotomy for cerebellar HB at another hospital 9 years ago. The tumor had not been completely resected, and the patient underwent follow-up at the hospital. However, the patient discontinued follow-up after the 3-year visit.
Figure 1:
(a) Gadolinium-enhanced magnetic resonance imaging (MRI) performed before the first surgery revealed a solid tumor in the right cerebellar hemisphere. (b) MRI taken 3 years after the first surgery showed a small enhanced nodule at the cerebellar surface. (c) Computed tomography at our hospital revealed a tumor recurrence. (d) MRI showing a cystic-solid tumor.
Tumor embolization was conducted under general anesthesia. Right vertebral angiography revealed a tumor receiving blood supply from the branches of the right SCA and PICA [
Figure 2:
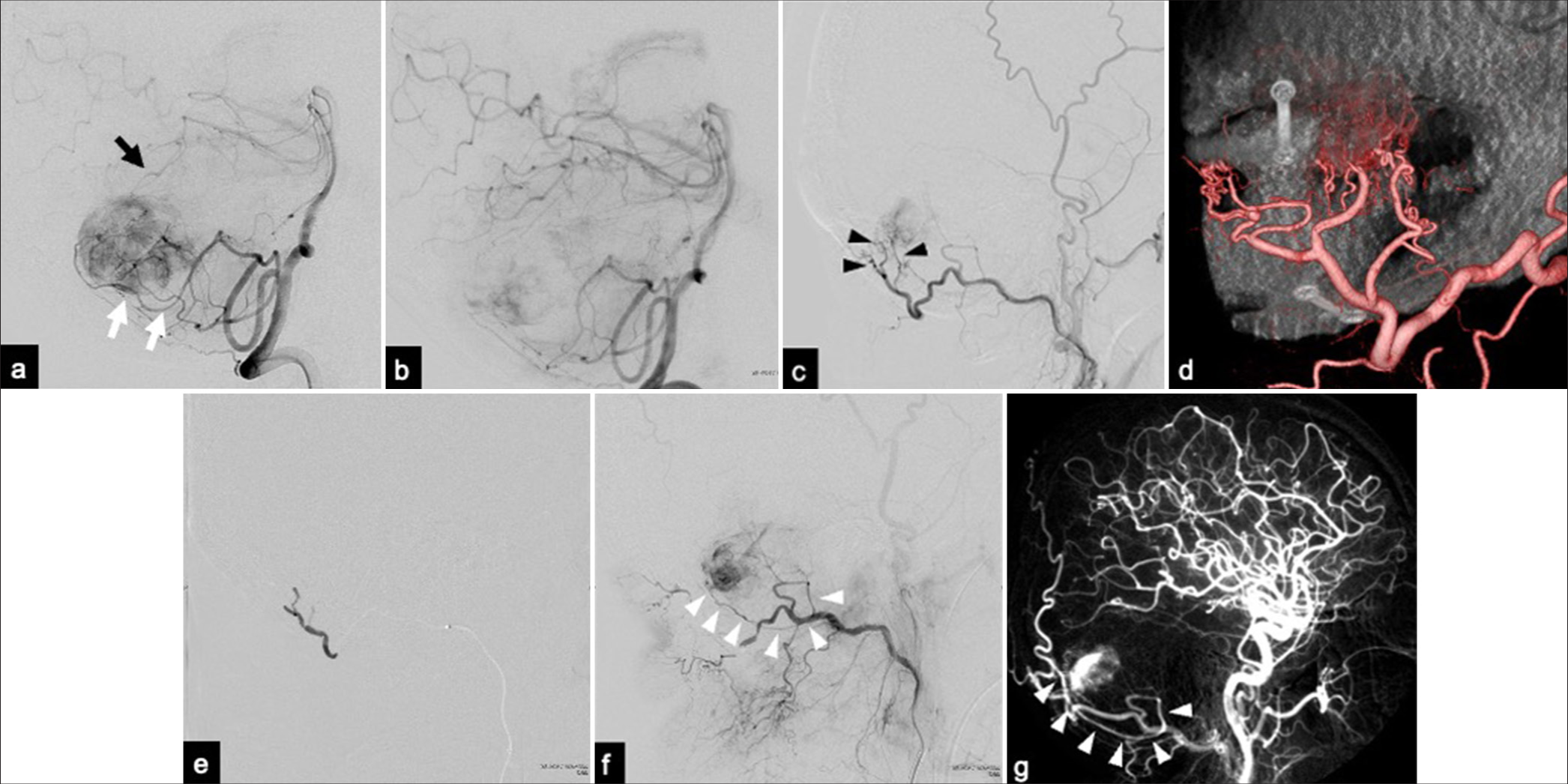
(a) Lateral view of the right vertebral angiography showing the tumor blush from the branches of the superior cerebellar artery (SCA) (black arrow) and posterior inferior cerebellar artery (PICA) (white arrows). (b) The tumor blush was reduced after embolization through the SCA and PICA. (c) Lateral view of the right external carotid angiogram showing the transosseous branches (TOBs) (black arrowheads) from the occipital artery (OA) as the feeding arteries. (d) Three-dimensional external carotid angiography revealing TOBs through the skull gap created by the previous craniotomy. (e) N-butyl-2-cyanoacrylate was injected into the OA near the origin of the TOBs, leading to feeder occlusion. (f) External carotid angiography after feeder occlusion revealed the mastoid branch from the OA as a tumor feeder (white arrowheads). (g) An angiogram obtained at a previous hospital revealed the mastoid branch, which was similar to the angiogram performed in our hospital (white arrowheads).
Postsurgical MRI revealed no remnant tumor [
DISCUSSION
HBs are benign intramedullary vascular tumors that are often situated near the pia mater. Therefore, they generally receive blood supply from the intracranial arteries, such as the branches from the PICA, AICA, and SCA; however, blood supply from the meningeal branches or arteries originating from ECA is relatively rare.[
The present case of recurrent cerebellar HB was rare because the BM and TOBs fed it from the OA. The BM from the OA was noted as a feeding artery before the first surgery performed 9 years before the present one. The tumor could not be completely resected during the first surgery, and the MB may have remained as one of the feeding arteries, facilitating tumor recurrence. TOBs have been speculated to develop and flow into the tumor through the skull gap during tumor regrowth. The TOBs in the present case were not “true” transosseous because the branches did not penetrate the skull; however, they passed through the skull gap created by craniotomy. Thus, these TOBs may be considered part of the meningeal branches in the present case.
With the development of endovascular treatment, preoperative embolization for HBs is considered an effective treatment to minimize intraoperative blood loss and increase complete resection rate and is now widely performed.[
The favorable outcome in our patient was attributed to complete resection. In general, reoperations are more complicated than initial operations due to adhesions and anatomical transformations that occur due to initial interventions. In this case requiring reoperation, complete resection was achieved with minimal bleeding despite strong adhesion. This was because bleeding from the tumor, as well as bleeding during craniotomy, was minimal. Considering that the TOBs developed with the tumor recurrence through the skull and dura, bleeding would have increased during craniotomy if TOBs had not been embolized. Thus, although TOBs are not the main feeders of HBs, embolization for TOBs can be safe and effective for craniotomy, dural incision, and tumor dissection, especially in recurrent HBs.
CONCLUSION
This is a rare case of recurrent HB with blood supply from the MB and TOBs from the OA. While preoperative embolization of branches from the intracranial arteries carries the risk of ischemic complications, embolization for TOBs is safe and effective for craniotomy and resection for recurrent HB.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
References
1. Ampie L, Choy W, Lamano JB, Kesavabhotla K, Kaur R, Parsa AT. Safety and outcomes of preoperative embolization of intracranial hemangioblastomas: A systematic review. Clin Neurol Neurosurg. 2016. 150: 143-51
2. Boutakioute B, Zouine Y, Chehboun A, Ouali M, Ganouni NC. Successful preoperative embolization of a cystic-solid variant of cerebellopontine angle hemangioblastoma. Radiol Case Rep. 2022. 17: 4799-803
3. Cui H, Zou J, Bao YH, Wang MS, Wang Y. Surgical treatment of solid hemangioblastomas of the posterior fossa: A report of 28 cases. Oncol Lett. 2017. 13: 1125-30
4. Hishikawa T, Sugiu K, Hiramatsu M, Haruma J, Tokunaga K, Date I. Nationwide survey of the nature and risk factors of complications in embolization of meningiomas and other intracranial tumors: Japanese registry of neuroendovascular therapy 2 (JR-NET2). Neuroradiology. 2014. 56: 139-44
5. Meena RK, Dhandapani S, Gupta V, Anirudh S, Chatterjee D. Solid hemangioblastoma in the cerebellopontine angle: Importance of external carotid blood supply with regard to the probable site of origin and preoperative embolization. Surg Neurol Int. 2016. 7: S1-4
6. Roberti F, Jones RV, Wright DC. Cranial nerve hemangioblastomas. Report of a rare case and review of literature. Surg Neurol. 2007. 67: 640-6
7. Sakamoto N, Ishikawa E, Nakai Y, Akutsu H, Yamamoto T, Nakai K. Preoperative endovascular embolization for hemangioblastoma in the posterior fossa. Neurol Med Chir (Tokyo). 2012. 52: 878-84
8. Sugiu K, Hishikawa T, Murai S, Takahashi Y, Kidani N, Nishihiro S. Treatment outcome of intracranial tumor embolization in Japan: Japanese registry of neuroendovascular therapy 3 (JR-NET3). Neurol Med Chir (Tokyo). 2019. 59: 41-7
9. Sultan A, Hassan T, Aboul-Enein H, Mansour O, Ibrahim T. The value of preoperative embolization in large and giant solid cerebellar hemangioblastomas. Interv Neuroradiol. 2016. 22: 482-8
10. Vargas-Urbina J, Crisanto-Silva JA, Vásquez-Perez C, DavilaAdrianzén A, Alcas-Seminario D, Lines-Aguilar W. Multimodal management of giant solid hemangioblastomas in two patients with preoperative embolization. Surg Neurol Int. 2024. 15: 144
11. Yamada SM, Ikeda Y, Takahashi H, Teramoto A, Yamada S. Hemangioblastomas with blood supply from the Dural arteries--two case reports. Neurol Med Chir (Tokyo). 2000. 40: 69-73