- Department of Anesthesia and Critical care, Government Medical College, Thiruvananthapuram, Kerala, India
- Department of Neuroanesthesia and Critical Care, Sree Chitra Tirunal Institute for Medical Sciences and Technology, Thiruvananthapuram, Kerala, India
- Department of Neurosurgery, Sree Chitra Tirunal Institute for Medical Sciences and Technology, Thiruvananthapuram, Kerala, India.
Correspondence Address:
Ajay Prasad Hrishi, Department of Neuroanesthesia and Critical Care, Sree Chitra Tirunal Institute for Medical Sciences and Technology, Thiruvananthapuram, Kerala, India.
DOI:10.25259/SNI_335_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Neeraja Ajayan1, Ajay Prasad Hrishi2, Manikandan Sethuraman2, Unnikrishnan Prathpadas2, Ranganatha Praveen2, Ganesh Divakar3. A prospective observational study to compare and evaluate delta down, aortic velocity time integral variability, and superior vena cava collapsibility index as predictors of fluid responsiveness in patients with supratentorial brain tumors undergoing elective neurosurgery. 05-Jul-2024;15:229
How to cite this URL: Neeraja Ajayan1, Ajay Prasad Hrishi2, Manikandan Sethuraman2, Unnikrishnan Prathpadas2, Ranganatha Praveen2, Ganesh Divakar3. A prospective observational study to compare and evaluate delta down, aortic velocity time integral variability, and superior vena cava collapsibility index as predictors of fluid responsiveness in patients with supratentorial brain tumors undergoing elective neurosurgery. 05-Jul-2024;15:229. Available from: https://surgicalneurologyint.com/surgicalint-articles/12980/
Abstract
Background: Patients undergoing surgical resection of brain tumors frequently exhibit a spectrum of hemodynamic fluctuations necessitating careful fluid management. This study aimed to evaluate the feasibility of dynamic predictors of fluid responsiveness, such as delta down (DD), aortic velocity time integral variability (VTIAoV), and superior vena cava collapsibility index (SVCCI), in patients undergoing neurosurgery for brain tumors.
Methods: In this prospective study, 30 patients scheduled to undergo elective neurosurgery for brain tumor resection were enrolled. Baseline measurements of vitals, anesthetic parameters, and study variables were recorded post-induction. Subsequently, patients received a fluid bolus of 10 mL/kg of colloid over 20 min, and measurements were repeated post-loading. Data were presented as mean ± standard deviation. The normally distributed continuous variables were compared using Student’s t-test, with P
Results: Of the 30 patients, 22 were identified as volume responders (R), while eight were non-responders (NR). DD >5 mmHg effectively distinguished between R and NR (P 38% differentiated R from NR (P 20% was also a good predictor (P
Conclusion: Our study revealed that most patients undergoing surgical resection of brain tumors exhibited fluid responsiveness. Among the variables assessed, SVCCI >38% emerged as an excellent predictor, followed by VTIAoV >20% and DD >5 mm Hg, for evaluating fluid status in this population.
Keywords: Aortic velocity time integral variability, Brain tumors, Delta down, Fluid responsiveness, Superior vena cava collapsibility index
INTRODUCTION
Patients presenting for neurosurgery are prone to major fluid shifts in the perioperative period due to a multitude of causative factors, including underlying occult cardiac dysfunction, neuroendocrine pathologies such as the syndrome of inappropriate antidiuretic hormone secretion, diabetes insipidus, and cerebral salt wasting.[
Predicting fluid responsiveness in these patients has been a significant challenge for anesthesiologists and intensivists. Due to the inherent difficulty in directly measuring intravascular volume, clinicians rely on various static and dynamic variables as indirect indicators of a patient’s fluid status. This study aimed to investigate fluid responsiveness in patients undergoing supratentorial brain tumor resection using dynamic indices obtained from hemodynamic monitors and echocardiography. Our objective was to evaluate the effectiveness of newer predictor indices such as delta down (DD), superior vena cava collapsibility index (SVCCI), and aortic velocity time integral variability (VTIAoV) in predicting fluid responsiveness in patients undergoing elective craniotomy for supratentorial brain tumor resection. In addition, we sought to validate the threshold for these predictor variables in discriminating responders (R) from non-responders (NR) within this population.
MATERIALS AND METHODS
This prospective observational study was conducted on patients undergoing craniotomy for the surgical resection of supratentorial brain tumors. Approval was obtained from the Institutional Ethics Committee (SCT/IEC-559/FEBRUARY-2014), and written informed consent was acquired from all participants. Thirty patients aged between 18 and 60 years, undergoing neurosurgery in the supine position for supratentorial brain tumor resection, were recruited. Exclusion criteria encompassed patients undergoing posterior fossa surgery, those with associated cardiac and pulmonary pathologies, surgeries performed in positions other than supine, and individuals with contraindications for transesophageal echocardiography (TEE) probe insertion.
Anesthesia protocol
In the operating room, standard American Society of Anesthesiologists monitors, such as electrocardiography, noninvasive BP, and pulse oximetry, were initiated, and peripheral intravenous access was established. Anesthesia was induced with propofol 2–3 mg/kg, fentanyl 2–3 μg/kg, along with vecuronium 0.1 mg/kg to facilitate tracheal intubation. Mechanical ventilation was initiated in a volume-controlled mode with a tidal volume of 8 mL/kg, and the respiratory rate was adjusted to achieve a partial pressure of carbon dioxide of 32–38 mmHg without positive end-expiratory pressure. Anesthesia was maintained using sevoflurane of 0.8–1 minimum alveolar concentration and a continuous infusion of fentanyl 1–2 μg/kg/h and atracurium 0.3 mg/kg/h. Under ultrasound guidance, arterial access was established in the radial artery, and a central venous catheter in the right internal jugular vein was placed. Additional monitoring of central venous pressure (CVP), end-tidal carbon dioxide, end-tidal anesthetic gas concentration, and ventilatory parameters was commenced. A forced-air warming system (Bair Hugger Warming system, Augustine Medical, Eden Prairie, MN, USA) was utilized, and the temperature was monitored using a nasopharyngeal probe. A TEE probe (GE Vivid 7 with 9T 4.0-10.0 MHz multiplane TEE probe, GE Healthcare, Wauwatosa, WI 53226, USA) was inserted before patient positioning for surgery. The acquisition of predictor variables is detailed below.
Study variables
DD
The maximal systolic pressure (SPmax), minimal systolic pressure (SPmin), and reference systolic pressure at the end of the expiratory pause (SPref) were measured from the waveform on the monitor (Philips Intellivue, MX700, Philips Medizin Systems, Germany). A mean of three recordings during three consecutive respiratory cycles was utilized for statistical analysis. DD was calculated as the difference between the systolic arterial pressure at the end of a 5-s respiratory pause immediately before lung inflation and its minimal value during one mechanical breath, that is, DD = SPref–SPmin. DD >5 mmHg has been demonstrated to differentiate fluid R from NR effectively.[
SVCCI
The superior vena cava (SVC) was visualized using the mid-esophageal bi-caval view. An anatomical M-Mode was utilized to measure the necessary diameters. The measured SVC diameters included the maximum diameter on expiration (SVCmax) and the minimum diameter on inspiration (SVCmin), which were assessed during the same respiratory cycle. The statistical analysis utilized the average of two values. The SVCCI was calculated using the formula: SVCCI = ([SVCmax–SVCmin]/[SVCmax]). A cutoff value of 38% for SVCCI was employed to distinguish between R and NR.[
VTIAoV and cardiac index
The left ventricular outflow tract (LVOT) and the opening of the aortic valve are visualized by obtaining the deep trans-gastric (TG-LAX) view. In this deep TG-LAX view, the aortic valve appears in the far field at the bottom of the display, with the LV outflow directed away from the transducer. Aortic velocity time integral (VTIAo) is calculated from the recorded velocity loops as a mean value obtained from three consecutive recordings. VTIAo variability (VTIAoV) is determined using the formula: VTIAoV = ([VTImax–VTI min]/VTI avg). VTIAoV >20% is the cutoff to distinguish fluid R from NR. Cardiac output (CO) is calculated in this view by multiplying the cross-sectional area of LVOT (CSA LVOT) × VTIAo × heart rate (HR). The cardiac index (CI) is computed by dividing CO by the body surface area.[
Study protocol
Baseline variables were documented following a 5-minute period of hemodynamic stability, defined as HR and systolic blood pressure (SBP) within ±5% after the initiation of anesthesia. Volume expansion was accomplished by fluid loading (FL) by administering 10 mL/kg of colloid solution (TetraHES, hydroxyethyl starch 130/0.4, Claris Otsuka, India) over 20 min. All parameters were reassessed post-FL. The anesthetic concentration and ventilator settings remained unchanged throughout the data acquisition period. Those patients exhibiting >15% variability in CI variability (CIV) following FL were defined as R, and those displaying CIV < 15% as NR.
Statistical analysis
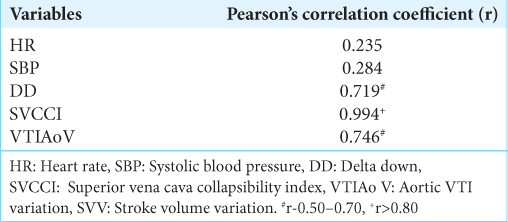
All statistical analyses were performed using the Statistical Package for the Social Sciences Inc., Chicago, IL, version 26.0. Continuous variables were presented as mean ± standard deviation or median (interquartile range) for skewed data, while categorical data were described using frequency, ratio, and percentage (%). The categorical variables were compared using the Chi-square test. Continuous variables were compared using a student’s t-test. All the statistical tests were carried out at a 5% level of significance, and a value <0.05 was considered significant. The correlation between the test variables (DD, SVCCI, and VTIAoV) and the gold standard for identifying fluid R and NR, namely, CIV, was assessed using Pearson’s correlation coefficient. A Pearson’s coefficient exceeding 0.8 indicated a strong correlation, while a value between 0.5 and 0.7 showed a good correlation. Pearson’s coefficient was preferred over receiver operating characteristic analysis due to the small size of the study population.
RESULTS
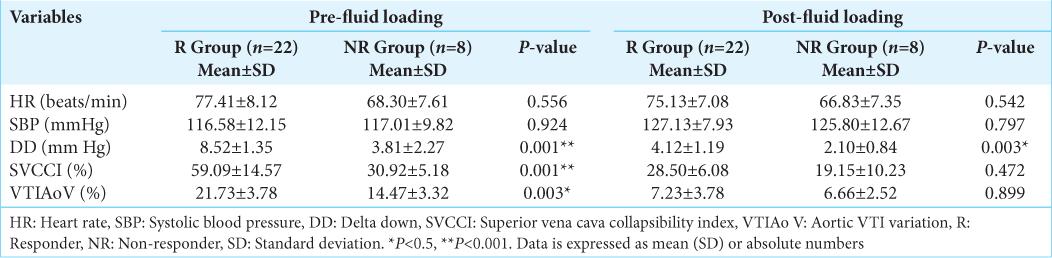
We recruited 30 subjects undergoing neurosurgery for brain tumor resection, and the study cohort comprised 16 males and 14 females. None of the patients required vasoactive drug therapy during the period of data acquisition. There were 22 volume R (73.33%) and 8 NR (26.67%). The clinical characteristics of R and NR were similar, and no difference was observed between these two groups in terms of anesthetic requirements and ventilatory parameters [
Our results showed that DD >5 mmHg efficiently differentiated the R from NR with a sensitivity and specificity of 94% and 80%, respectively. FL in patients suspected to be hypovolemic and who were later diagnosed as responders showed a baseline DD below the threshold value of 5 mmHg (P < 0.001) [
SVCCI of >38% was 100% sensitive and 100% specific in detecting the volume status and in detecting the volume status of our patients. The mean value of SVCCI was significantly elevated above the 38% cutoff (P < 0.001) in the responder group, whereas it was below the threshold value in the non-responder population. Responders had a significant decrease in SVCCI post-FL, demonstrating these subjects’ positive response to fluid therapy [
A VTIAo variability threshold value of >20% had a sensitivity and specificity of 100% and 92%, respectively, in differentiating the R from NR. Responders had a baseline VTIAoV >20% (P < 0.05). Post FL, the responders had a sharp decline in the VTIAoV to <20% in contrast to the NR, demonstrating that the subjects were grossly fluid deficient at the beginning of the procedure. VTIAoV had a good correlation with the cardiac index variation; r = 0.746 [
DISCUSSION
The objective of our study was to evaluate the feasibility of DD, SVCCI, and VTIAoV as predictors of fluid responsiveness and to validate the threshold values for distinguishing fluid R from NR in patients undergoing neurosurgery for brain tumors. We found no difference in basic hemodynamic parameters, such as HR and BP, between the R and NR groups. Even post-FL, these indices remained essentially unchanged; they also showed poor correlation with CIV, suggesting that they are poor indices of volume status. Hence, our findings indicate that these variables are unreliable for assessing and managing volume status in patients with brain tumors.
Considerable evidence indicates that static indices such as CVP, pulmonary artery occlusion pressure, and variables derived from echocardiographic assessments such as right atrial pressure, left ventricular (LV) end-diastolic volume, and area are inadequate for accurately assessing changes in ventricular preload, thus rendering them poor predictors of fluid responsiveness.[
Recent studies suggest that ventilation-induced variations in arterial pulse pressure waveform can accurately predict fluid responsiveness despite minor limitations.[
We demonstrated that DD, with a threshold of 5 mmHg, is a dependable predictor for assessing volume status, effectively distinguishing between R and NR groups within the brain tumor population. It showed a good correlation with CIV post-FL, accurately predicting fluid responsiveness. Our finding is similar to previous studies, which showed that DD >5 mmHg could differentiate between R and NR. They also observed a consistent decrease in DD below threshold values post-FL in patients with hypovolemia, a pattern seen in our study.[
In our study, TEE was utilized to obtain dynamic variables such as SVC diameters, aortic VTI, and LVOT, based on which we calculated the SVCCI and VTIAoV. To the best of our knowledge, no study has investigated the reliability of SVCCI and VTIAoV in predicting volume responsiveness in patients with brain tumors during the intraoperative period. We regarded VTIAoV as a proxy measure for changes in preload and LV contractile function. TEE facilitated simultaneous quantification of changes in loading conditions, CO, and diastolic function in our subset of patients with intracranial tumors, who exhibit dynamic variability in cardiac compliance.[
The assessment of the SVC diameter and its derived metric, the SVCCI, revealed its effectiveness as a predictor of fluid status within this patient subset. The R and NR groups had smaller SVC diameters before FL, which increased post-FL. SVCCI within the R group significantly exceeded the 38% threshold before FL and after that decreased post-FL, thus confirming its efficacy as a predictor of fluid responsiveness. Variations in SVCCI correlated strongly with CIV, even among NR, in our study population. Vieillard-Baron et al. found that in patients with sepsis, SVCCI >38% effectively discriminated between R and NR with high sensitivity and specificity. They advocated routine SVCCI measurement in septic shock patients as an accurate indicator of fluid responsiveness.[
We observed that VTIAo variability exceeding 20% has good sensitivity and specificity in distinguishing between R and NR. The mean value within the responder group significantly surpassed the 20% threshold, which notably decreased below the threshold post-FL, indicating its predictive capacity for fluid status. Moreover, it displayed a good correlation with CIV, affirming its efficacy as a predictor. Our analysis demonstrated that VTIAoV behaved similarly in the brain tumor population compared to prior studies, validating its efficacy as a predictor in the brain tumor population. Feissel et al. found that in mechanically ventilated patients with septic shock, aortic peak velocity (Vpeak) variation >12% effectively differentiated R from NR, with high predictive value.[
The findings of this study shed light on critical aspects of fluid therapy in patients with brain tumors undergoing surgical intervention. This insight will assist in devising meticulous fluid management strategies to enhance outcomes in individuals with brain tumors, thus mitigating the risks of cerebral edema, perioperative strokes, and lung complications.[
Limitations
We did not validate our variables against thermodilution techniques with a pulmonary artery catheter, which is currently considered the gold standard for CO measurement. However, studies have shown a good correlation between CO measured by TEE and measurements obtained through the pulmonary artery catheter.[
CONCLUSION
Our study revealed that most patients undergoing surgical resection of supratentorial brain tumors exhibited fluid responsiveness, even though conventional hemodynamic indicators such as HR and BP were within normal limits. Among these variables, SVCCI >38% has the most robust predictive value, followed by VTIAoV >20% and DD >5 mmHg in evaluating fluid status in this patient cohort. In the current era of point-of-care ultrasound-guided hemodynamic management, our study underscores the relevance of incorporating echocardiographic variables and arterial waveform-derived indices to predict fluid responsiveness in these patients.
Ethical approval
The research/study approved by the Institutional Review Board at SREE CHITRA TIRUNAL INSTITUTE FOR MEDICAL SCIENCES AND TECHNOLOGY, number SCT/IEC-559/FEBRUARY-2014, dated March 03, 2014.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Belloni L, Pisano A, Natale A, Piccirillo MR, Piazza L, Ismeno G. Assessment of fluid-responsiveness parameters for off-pump coronary artery bypass surgery: A comparison among LiDCO, transesophageal echocardiography, and pulmonary artery catheter. J Cardiothorac Vasc Anesth. 2008. 22: 243-8
2. Bubenek-Turconi ŞI, Hendy A, Băilă S, Drăgan A, Chioncel O, Văleanu L. The value of a superior vena cava collapsibility index measured with a miniaturized transoesophageal monoplane continuous echocardiography probe to predict fluid responsiveness compared to stroke volume variations in open major vascular surgery: A prospective cohort study. J Clin Monit Comput. 2020. 34: 491-9
3. Byon HJ, Lim CW, Lee JH, Park YH, Kim HS, Kim CS. Prediction of fluid responsiveness in mechanically ventilated children undergoing neurosurgery. Br J Anaesth. 2013. 110: 586-91
4. Coudary A, Romand JA, Treggiari M, Bendjelid K. Fluid responsiveness in spontaneously breathing patients: A review of indexes used in intensive care. Crit Care Med. 2005. 33: 275762
5. Charron C, Fessenmeyer C, Cosson C, Mazoit JX, Herbert JL, Benhamou D. The influence of tidal volume on the dynamic variables of fluid responsiveness in critically ill patients. Anesth Analg. 2006. 102: 1511-7
6. Cherpanath TG, Geerts BF, Lagrand WK, Schultz MJ, Groeneveld AB. Basic concepts of fluid responsiveness. Neth Heart J. 2013. 21: 530-6
7. Deflandre E, Bonhomme V, Hans P. Delta down compared with delta pulse pressure as an indicator of volemia during intracranial surgery. Br J Anaesth. 2008. 100: 245-50
8. Desai N, Garry D. Assessing dynamic fluid-responsiveness using transthoracic echocardiography in intensive care. BJA Educ. 2018. 18: 218-26
9. Feissel M, Michard F, Mangin I, Ruyer O, Faller JP, Teboul JL. Respiratory changes in aortic blood velocity as an indicator of fluid responsiveness in ventilated patients with septic shock. Chest. 2001. 119: 867-73
10. Fugate JE. Complications of neurosurgery. Continuum (Minneap Minn). 2015. 21: 1425-44
11. Hasanin A, Zanata T, Osman S, Abdelwahab Y, Samer R, Mahmoud M. Pulse pressure variation-guided fluid therapy during supratentorial brain tumour excision: A randomized controlled trial. Open Access Maced J Med Sci. 2019. 7: 2474-9
12. Huang CC, Fu Jy, Hu HC, Kao KC, Chen NH, Hsieh MJ. Prediction of fluid responsiveness in acute respiratory distress syndrome patients ventilated with low tidal volume and high positive end-expiratory pressure. Crit Care Med. 2008. 36: 2810-6
13. Hrishi AP, Sethuraman M, Menon G. Quest for the holy grail: Assessment of echo-derived dynamic parameters as predictors of fluid responsiveness in patients with acute aneurysmal subarachnoid hemorrhage. Ann Card Anaesth. 2018. 21: 243-8
14. Kim SH, Kim M, Lee JH, Cho SH, Chae WS, Cannesson M. Current practice in hemodynamic monitoring and management in high-risk surgery patients: A national survey of Korean anesthesiologists. Korean J Anesthesiol. 2013. 65: 19-32
15. Le Guen M, Le Gall-Salaun A, Josserand J, Gaudin de Vilaine A, Viquesnel S, Muller D. Goal-directed fluid therapy and major postoperative complications in elective craniotomy. A retrospective analysis of a before-after multicentric study. BMC Anesthesiol. 2023. 23: 11
16. Li Y, Jiang L, Wang L, Dou D, Feng Y. Evaluation of fluid responsiveness with dynamic superior vena cava collapsibility index in mechanically ventilated patients. Perioper Med. 2023. 12: 10
17. Luo J, Xue J, Liu J, Liu B, Liu L, Chen G. Goal-directed fluid restriction during brain surgery: A prospective randomized controlled trial. Ann Intensive Care. 2017. 7: 16
18. Manea MM, Comsa M, Minca A, Dragos D, Popa C. Brain-heart axis-review article. J Med Life. 2015. 8: 266-71
19. McCluskey SA, Karkouti K, Wijeysundera D, Minkovich L, Tait G, Beattie WS. Hyperchloremia after noncardiac surgery is independently associated with increased morbidity and mortality: A propensity-matched cohort study. Anesth Analg. 2013. 117: 412-21
20. Michard F. Changes in arterial pressure during mechanical ventilation. Anesthesiology. 2005. 103: 419-28
21. Monnet X, Shi R, Teboul JL. Prediction of fluid responsiveness. What’s new? Ann Intensive Care. 2022. 12: 46
22. Pérez-Manjarrez A, García-Cruz E, Gopar-Nieto R, JiménezRodríguez GM, Lazcano-Díaz E, Rojas-Velasco G. Usefulness of the velocity-time integral of the left ventricular outflow tract variability index to predict fluid responsiveness in patients undergoing cardiac surgery. Echo Res Pract. 2023. 10: 9
23. Ramakumar N, Gupta P, Arora R, Agrawal S. A prospective exploratory study to assess echocardiographic changes in patients with supratentorial tumors-effect of craniotomy and tumor decompression. Surg Neurol Int. 2023. 14: 166
24. Ryu T. Fluid management in patients undergoing neurosurgery. Anesth Pain Med. 2021. 16: 215-24
25. Sabatier C, Monge I, Maynar J, Ochagavia A. Assessment of cardiovascular preload and response to volume expansion. Med Intensiva. 2012. 36: 45-55
26. Slama M, Masson H, Teboul JL, Arnout ML, Susic D, Frohlich E. Respiratory variations of aortic VTI: A new index of hypovolemia and fluid responsiveness. Am J Physiol Heart Circ Physiol. 2002. 283: H1729-33
27. Sundaram SC, Salins SR, Kumar AN, Korula G. Intra-operative fluid management in adult neurosurgical patients undergoing intracranial tumour surgery: Randomised control trial comparing pulse pressure variance (PPV) and central venous pressure (CVP). J Clin Diagn Res. 2016. 10: UC01-5
28. Vieillard-Baron A, Augarde R, Prin S, Page B, Beauchet A, Jardin F. Influence of superior vena caval zone condition on cyclic changes in right ventricular outflow during respiratory support. Anesthesiology. 2001. 95: 1083-8
29. Wagner JG, Leatherman W. Right ventricular end-diastolic volume as a predictor of the hemodynamic response to a fluid challenge. Chest. 1998. 113: 1048-54
30. Wang J, Zhou D, Gao Y, Wu Z, Wang X, Lv C. Effect of VTILVOT variation rate on the assessment of fluid responsiveness in septic shock patients. Medicine (Baltimore). 2020. 99: e22702
31. Wrzosek A, Jakowicka-Wordliczek J, Zajaczkowska R, Serednicki WT, Jankowski M, Bala MM. Perioperative restrictive versus goal-directed fluid therapy for adults undergoing major non-cardiac surgery. Cochrane Database Syst Rev. 2019. 12: CD012767
32. Wu CY, Lin YS, Tseng HM, Cheng HL, Lee TS, Lin PL. Comparison of two stroke volume variation-based goal-directed fluid therapies for supratentorial brain tumour resection: A randomized controlled trial. Br J Anaesth. 2017. 119: 934-42