- Department of Neurosurgery, Jessa Hospital, Hasselt, Belgium
- Department of Neurosciences, Faculty of Medicine, University of Hasselt, Hasselt, Belgium
- Department of Pathology, Jessa Hospital, Hasselt, Belgium
- Limburg Clinical Research Centre (LCRC), University of Hasselt, Hasselt, Belgium
Correspondence Address:
Wouter Deconinck, Department of Neurosurgery, Jessa Hospital, Hasselt, Belgium.
DOI:10.25259/SNI_640_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Wouter Deconinck1, Sven Bamps2, Thomas Steelandt3, Maarten Wissels4, Mark Plazier2, Eric Put4, Salah-Eddine Achahbar4, Steven Vanvolsem4, Sacha Meeuws4, Sam Klein4, Gert Roosen4. A rare case of spinal myeloid sarcoma. 15-Nov-2024;15:415
How to cite this URL: Wouter Deconinck1, Sven Bamps2, Thomas Steelandt3, Maarten Wissels4, Mark Plazier2, Eric Put4, Salah-Eddine Achahbar4, Steven Vanvolsem4, Sacha Meeuws4, Sam Klein4, Gert Roosen4. A rare case of spinal myeloid sarcoma. 15-Nov-2024;15:415. Available from: https://surgicalneurologyint.com/surgicalint-articles/13230/
Abstract
Background: Myeloid sarcoma (MS), a rare extramedullary tumor composed of myeloid blast cells, is classified by the World Health Organization as a subtype of acute myeloid leukemia (AML). Isolated, primary, or nonleukemic MS has an incidence of 2/1,000,000 adults and constitutes only 0.7% of all AML cases. MS presentations vary widely, with spinal involvement being rare.
Case Description: A-year-old male presented with interscapular pain radiating to the right upper arm/neck but was neurologically intact. Once diagnosed with isolated spinal MS, he underwent a surgical decompression followed by local irradiation, systemic chemotherapy, and bone marrow transplantation. Eight months postoperatively, however, he experienced a graft-versus-host rejection and required additional therapies.
Conclusion: Establishing the diagnosis of MS is challenging and typically requires histological confirmation (i.e., the presence of myeloblasts and granulocytic cells). However, optimal treatment strategies remain elusive; despite radiation, chemotherapy, bone marrow transplant/other local therapies, the overall long-term prognosis for MS remains poor.
Keywords: Compression fracture, Myeloid sarcoma, Polycythemia vera, Spinal tumor, Thoracic spine
INTRODUCTION
Unlike other lymphoproliferative neoplasms, myeloid neoplasms form masses outside of the bone marrow and invade different organ systems.[
CASE
Clinical history
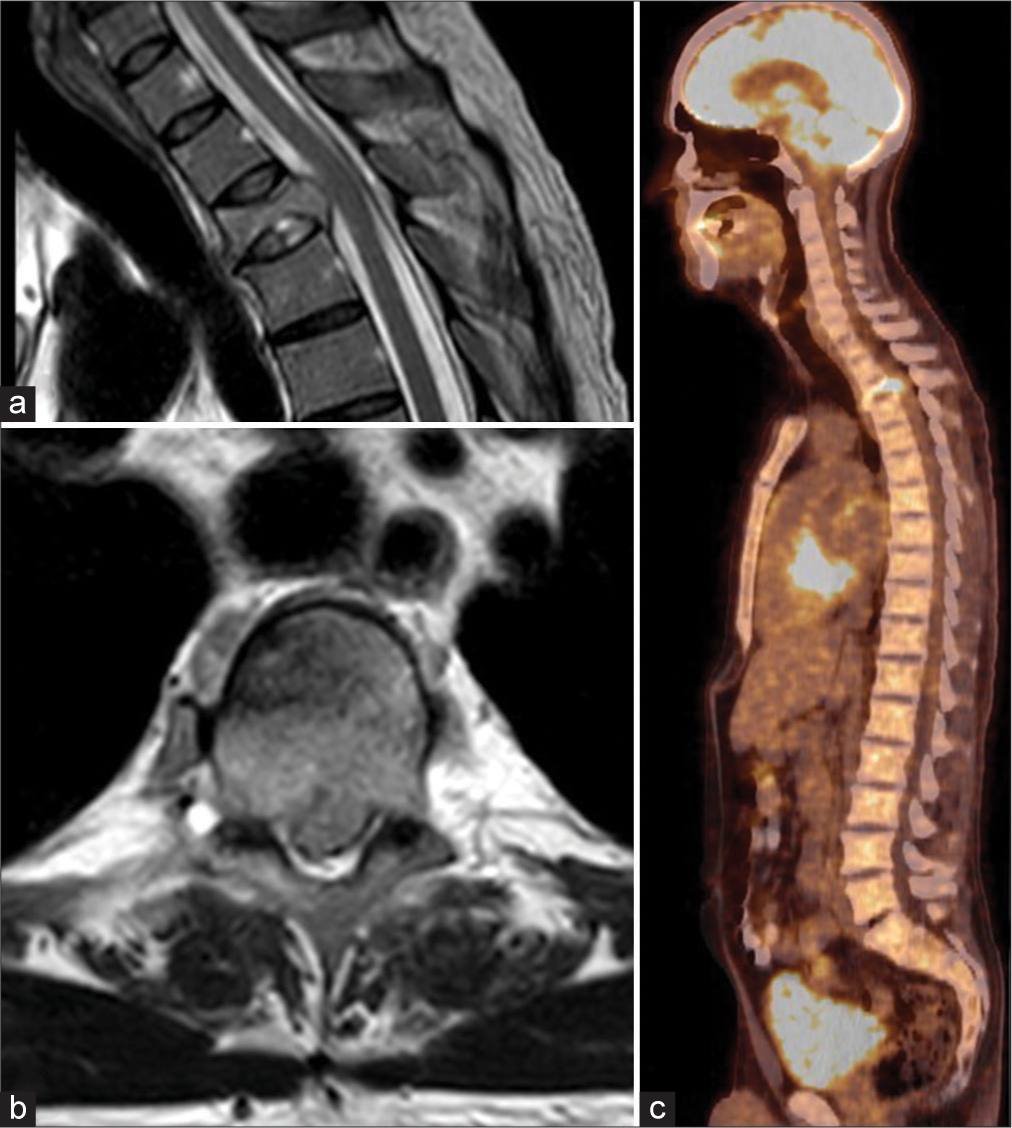
A 54-year-old male presented with interscapular pain radiating to the right upper arm/neck of 2 months’ duration but was otherwise neurologically intact. He had a known history of myelofibrotic polycythemia vera (JAK-2 mutation) previously treated with ruxolitinib (Jakavi®). His thoracic magnetic resonance imaging (MRI) showed a T3 “pathological” (i.e., signal alterations of the bone) vertebral body compression fracture, accompanied by tumor extending into the right pedicle and epidural space contributing to mild cord compression (i.e., but no hyperintense cord signal). The lesion showed high metabolic activity on the fluorodeoxyglucose-positron emission tomography scan; notably, no other lesions were identified [
Figure 1:
(a) Sagittal T2-weighted image of the upper thoracic spine, showing an impression fracture of the Th3 vertebral body, with pathological signal alteration, bulging into the spinal canal, with slight compression of the dural sac. There is no myelomalacia. (b) Axial T2-weighted image of the affected Th3 vertebra. (c) Fluorodeoxyglucose-positron emission tomography/computed tomography scan in the sagittal plane shows increased tracer captation of the Th3 vertebral body; there are no suspicious lesions elsewhere.
Bone marrow biopsy confirming malignant MS
Given the assumption that the lesion was malignant, the patient underwent bone marrow biopsies; they showed hypercellularity in the myeloid and megakaryocytic cell line consistent with MS. There was also grade 1 myelofibrosis and nonspecific finding for two TP53 gene mutations but no evidence for monoclonal gammopathy or proliferation of myeloid blast cells (CD34 staining).
Surgery
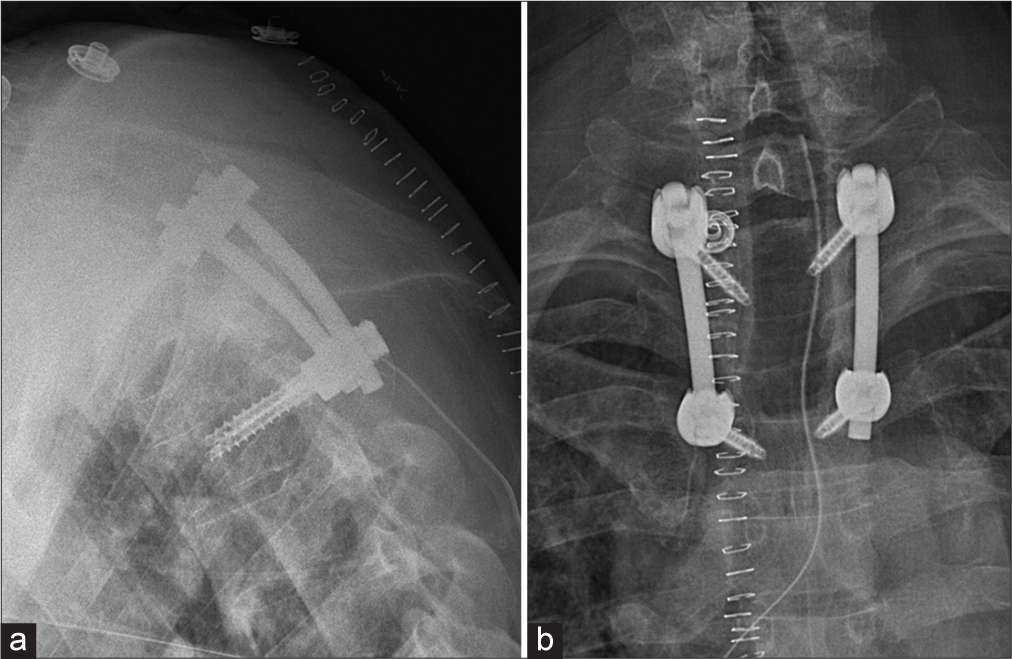
Utilizing CT-guided neuronavigation, the patient underwent decompressive surgery-tumor biopsy plus T2–T4 pedicle screw fusion.
Pathology
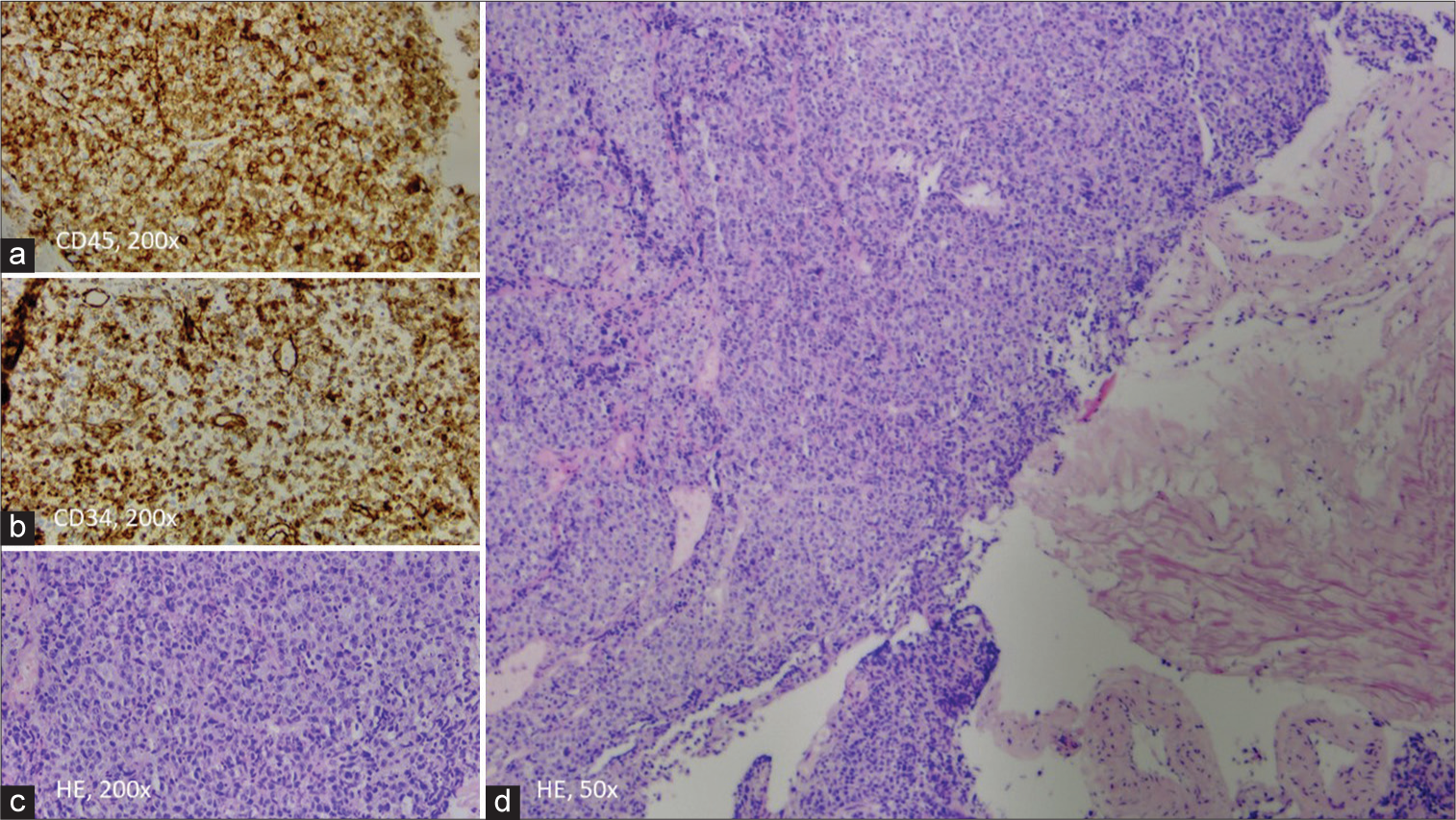
Microscopy showed diffuse proliferation of poorly differentiated tumor cells consistent with the diagnosis of MS. These cells had scant to moderate pale cytoplasm and vesicular nuclei. Immunohistochemical characterization showed that these tumor cells were negative for SOX10, CD138, INSM1, synaptophysin, chromogranin A, CK20, TTF1, S100, SMA, Desmine, Myogenine, CD3, CD20, CD117, CD68, TDT, CD1a, PAX5, CD30, MUM1, EBV ISH, CD21, and myeloperoxidase (MPO). There was expression of CD45, CD34, CD43, and paranuclear AE1/AE3 staining. Both morphology and immunohistochemistry were most consistent with myeloid blasts, rendering the diagnosis of a spinal MS [
Figure 2:
(a and b) Expression of CD45 and CD34 in tumor cells. (c) ×200 magnification of a monomorphic of immature myeloid cells with pale cytoplasm and vesicular nuclei. (d) Low-power view (×50 magnification) of the diffuse growing tumor, next to fragments of the ligamentum flavum. (HE=Haematoxylin and eosin stain)
Adjuvant treatment
The patient received local irradiation therapy (5 × 4 Gy) followed by chemotherapy and an allogenic bone marrow transplant. The latter was later complicated by grade 3 graft-versus-host rejection that was successfully treated with corticosteroids, ruxolutinib, and beclomethasone dipropionate. Months later, there was another complication of EBV-related posttransplant lymphoproliferative disease stage 3, for which the patient was treated with rituximab. Surgical follow-up at the 8-month interval was good with stable findings on plain radiograph. There was no further collapse of the vertebral body Th3 [
DISCUSSION
MS is an uncommon extramedullary malignant myeloid cell tumor. The disease is often associated with AML.[
Histological criteria of MS
The definitive diagnosis of MS is based primarily on histological examination and immunohistochemistry. Microscopically, MS typically shows a diffuse infiltrate of immature myeloid cells with variable degrees of maturation. Immunohistochemical stains play a crucial role in confirming the myeloid lineage of the neoplastic cells. Markers such as MPO, CD34, CD117, CD68, and lysozyme are commonly used in the diagnostic workup. Flow cytometry and molecular studies may also be helpful, particularly in cases with ambiguous histological features and immunohistochemical phenotypes.[
MRI of MS
MRI is the preferred modality for diagnosing MS. These lesions typically appear isointense to muscle on T1-weighted images and hyperintense on T2-weighted images. Gadolinium-enhanced MRI scans can provide additional information about the vascularity and extent of these lesions.[
Treatment modalities
Patients with MS involving the spinal epidural region often present with symptoms such as back pain, weakness, sensory deficits, or bowel and bladder dysfunction. Rapid progression of neurological deficits may occur, which emphasizes the need for early intervention to prevent irreversible sequelae.[
Radiation therapy
The role of radiotherapy in the management of MS is not well-defined and is often reserved for patients who are not candidates for intensive chemotherapy or consolidation therapy following systemic treatment. Radiation therapy can achieve local disease control and palliation of symptoms, particularly in cases of isolated MS involving bony structures or other sites not amenable to surgical resection.[
Surgery
Surgical resection may be considered in selected cases of MS.[
Prognosis
The prognosis of MS varies widely dependent on the patient’s age, general health status, extent of disease, and response to treatment.[
CONCLUSION
A 54-year-old male with MS involving the T3 vertebral body underwent a decompression/T2–T4 fusion, followed by radiation, chemotherapy, and a bone marrow transplant. When the lesion recurred 8 months later, additional adjuvant therapies were warranted.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Bai CR, Li X, Wang JS, Li JJ, Liu N, Fei Q. Diagnosis and surgical treatment of primary isolated aggressive lumbar myeloid sarcoma: A rare case report and review of the literatures. BMC Musculoskelet Disord. 2021. 22: 220
2. Bhandohal JS, Moosavi L, Garcia-Pacheco I, Yakoub G, Polineni RD, Cobos E. Isolated myeloid sarcoma of lumbar spine without bone marrow involvement: A rare case report and treatment dilemma. AME Case Rep. 2021. 5: 27
3. Chakraborty S, Chandra M, Ghosh J, Sarkar A. Myeloid sarcoma presenting with quadriparesis: A difficult journey through COVID times-lessons learned. Bengal Phys J. 2021. 8: 15-6
4. Fujikawa T, Kishimoto K, Inoue S, Nishimura A, Tojo R, Uemura S. Epidural spinal cord compression as the presenting manifestation of acute myeloid leukemia: A case report and literature review. Intern Med. 2023. 62: 453-7
5. Han S, Li Y, Niu T, Wang X, Li Z, Ren X. Granulocytic sarcoma causing long spinal cord compression: Case report and literature review. J Spinal Cord Med. 2022. 45: 481-5
6. Patel TD, Uzoaru I, Sutton BP, Arnold PM. Myeloid sarcoma of the thoracic spine: A case report. Surg Neurol Int. 2023. 14: 35
7. Shah K, Panchal H, Patel A. Spine myeloid sarcoma: A case series with review of the literature. South Asian J Cancer. 2021. 10: 251-4
8. Siddiqui H, Osmani AH. Spinal myeloid sarcoma in a nonleukaemic patient. J Co ll Physicians Surg Pak. 2024. 34: 118-9
9. Yang B, Yang C, Fang J, Yang J, Xu Y. Clinicoradiological characteristics, management and prognosis of primary myeloid sarcoma of the central nervous system: A report of four cases. Oncol Lett. 2017. 14: 3825-31