- Department of Intensive Care, Columbia Asia Hospital, Bengaluru, Karnataka, India.
- Department of Neurology, Columbia Asia Hospital, Bengaluru, Karnataka, India.
- Department of Haematology Columbia Asia Hospital, Bengaluru, Karnataka, India.
- Department of Neurosurgery, Columbia Asia Hospital, Bengaluru, Karnataka, India.
Correspondence Address:
Pradeep Rangappa, Department of Intensive Care, Columbia Asia Hospital, Bengaluru, Karnataka, India.
DOI:10.25259/SNI_689_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Raghavendra Kotal1, Ipe Jacob1, Pradeep Rangappa1, Karthik Rao1, Guruprasad Hosurkar2, Satish Kumar Anumula3, Avinasha M. Kuberappa4. A rare case of vaccine-induced immune thrombosis and thrombocytopenia and approach to management. 16-Aug-2021;12:408
How to cite this URL: Raghavendra Kotal1, Ipe Jacob1, Pradeep Rangappa1, Karthik Rao1, Guruprasad Hosurkar2, Satish Kumar Anumula3, Avinasha M. Kuberappa4. A rare case of vaccine-induced immune thrombosis and thrombocytopenia and approach to management. 16-Aug-2021;12:408. Available from: https://surgicalneurologyint.com/surgicalint-articles/11048/
Abstract
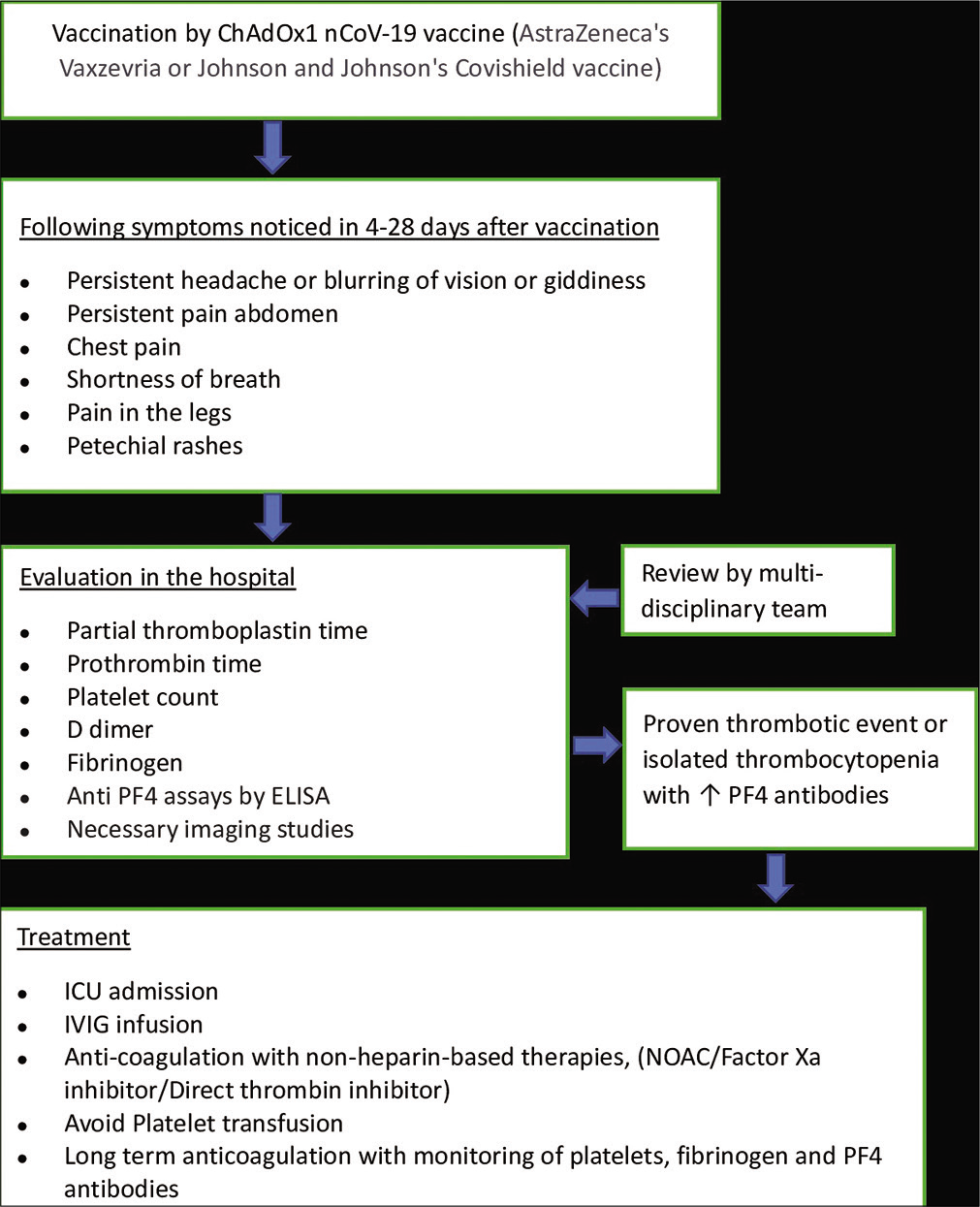
Background: The use of the COVID-19 vaccines Vaxzevria from AstraZeneca and Covishield from Janssen has been associated with sporadic reports of thrombosis with thrombocytopenia, a complication referred to as vaccine-induced immune thrombotic thrombocytopenia (VITT) or vaccine-induced prothrombotic immune thrombocytopenia. It presents commonly as cerebral sinus venous thrombosis (CSVT), within 4–30 days of vaccination. Females under 55 years of age are considered to be especially at high risk. Mortality up to 50% has been reported in some countries. Identification of early warning signs and symptoms with prompt medical intervention is crucial.
Case Description: We report here a case of VITT in a young female who presented 11 days after receiving the first dose of the Covishield vaccine, with severe headache and hemiparesis. She was diagnosed with CSVT with a large intraparenchymal bleed, requiring decompressive craniectomy and extended period on mechanical ventilation.
Conclusion: The patient was successfully treated with intravenous immunoglobulin and discharged after 19 days in ICU. Although she was left with long-term neurological deficits, an early presentation and a multidisciplinary approach to management contributed toward a relatively short stay in hospital and avoided mortality.
Keywords: Autoimmune heparin-induced thrombocytopenia, Cerebral venous sinus thrombosis, Platelet-factor 4 antibodies, Vaccine-induced immune thrombosis and thrombocytopenia, Vaccine-induced prothrombotic immune thrombocytopenia
INTRODUCTION
Vaccine-induced immune thrombotic thrombocytopenia (VITT)[
CASE REPORT
The patient is a 32-year-old female who presented with 3 days of headache associated with blurring of vision and giddiness. She also had weakness on the left upper and lower limb from 1 day. The family reported history of the patient receiving her first dose of Covishield vaccine 11 days before admission. She was unmarried, an active cigarette smoker, with no history of comorbidities or medications, nor any similar episodes in the past.
On examination, she was conscious and oriented, with heart rate of 76/min, respiratory rate of 22/min, blood pressure of 110/70 mmHg, oxygen saturation of 94% on room air, a normal Glasgow Coma Scale score, and normal pupils. Limb power was 2/5 and 3/5 on the left upper and lower limbs, respectively. A magnetic resonance imaging (MRI) of brain [
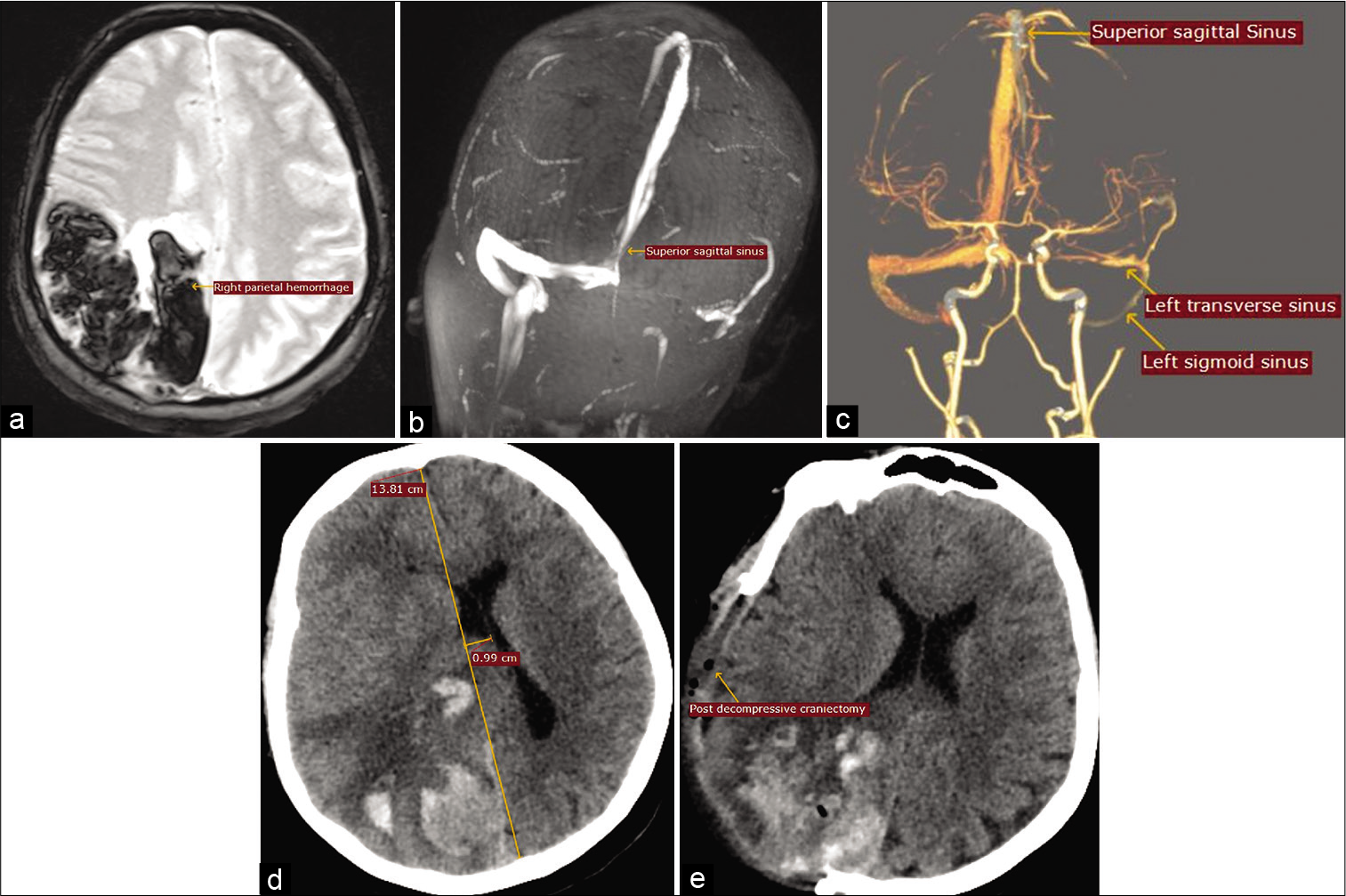
Figure 1:
(a) MRI brain T2-weighted-GRE axial view showing right parietal hemorrhage. (b) MR venogram 2D TOF MIP coronal view showing complete thrombosis of the left transverse and sigmoid sinuses and partial thrombosis of superior sagittal sinus. (c) CT venogram 3D volume rendering technique showing complete thrombosis of the left transverse and sigmoid sinuses and partial thrombosis of superior sagittal sinus. (d) CT brain axial view showing increase in midline shift. (e) CT axial view after right parietal decompression craniectomy.
On day 2 in ICU, her sensorium acutely deteriorated and a CT brain showed significant increase in the right parietal hematoma and worsening midline shift of 10 mm [
She underwent percutaneous tracheostomy on day 5 of admission, anticipating prolonged need for airway protection due to slow neurological recovery. Her sensorium started improving by day 6 and she was gradually weaned off ventilator. By day 10, she was conscious, oriented, breathing on room air through the tracheostomy, and communicating by writing. Her left lower limb power had improved to 4/5 although upper limb remained weak with power 3/5. By day 17, the tracheostomy was decannulated after assessing her cough and swallowing reflexes. She was monitored for another 2 days and discharged with home neurorehabilitation services, on tablet dabigatran 150 mg twice daily until the next review.
DISCUSSION
Worldwide, data on thrombotic events following COVID vaccination continues to evolve but initial studies show that the incidence of VITT varies widely according to age, gender, type and timing of vaccine, and across different geographies.[
Symptoms are mostly neurological, as in the present case, including severe and persistent headache, blurred vision, vomiting, seizures, focal neurological deficits, and altered sensorium.[
The underlying pathophysiology resembles that of heparin-induced thrombocytopenia (HIT), a prothrombotic disorder resulting from IgG-specific antibodies against platelet-factor 4 (PF4)-heparin complexes, causing platelet activation through the FcγRIIA receptor on the platelets.[
The British Society for Haematology[
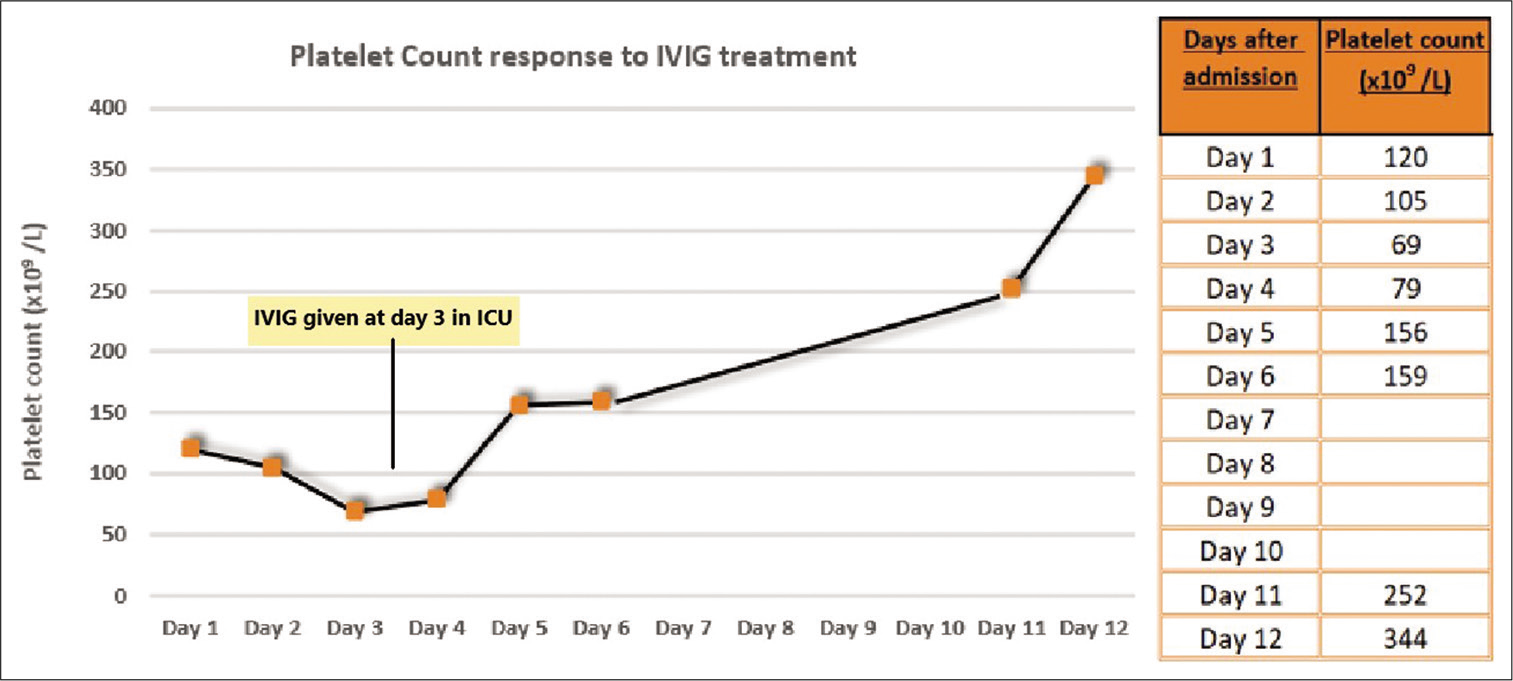
Treatment parallels that of autoimmune HIT and mainly involves IVIG 1 g/kg once daily for 2 days, which improves the platelet count within days, as seen in our patient, by limiting antibody-mediated platelet clearance and platelet activation by immune complexes.[
CONCLUSION
Although COVID vaccination can lead to this very rare, life-threatening thrombotic complication, most regulatory bodies advice that the benefits far outweigh the risks. Awareness of warning signs after vaccination and early medical treatment at a multidisciplinary specialty center, as well as modification of prothrombotic risk factors would help in preventing high morbidity and mortality.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. An Advisory Committee Statement (ACS) National Advisory Committee on Immunization (NACI) Recommendations on the use of COVID-19 Vaccines. Available from: https://www.canada.ca/en/public-health/services/immunization/national-advisory-committee-on-immunization-naci/recommendations-use-covid-19-vaccines.html [Last accessed on 2021 Jul 16].
2. Bikdeli B, Chatterjee S, Arora S, Monreal M, Jimenez D, Krumholz HM. Cerebral venous sinus thrombosis in the us population, after adenovirus-based SARS-CoV-2 vaccination, and after COVID-19. J Am Coll Cardiol. 2021. 78: 408-11
3. Cattaneo M. Thrombosis with thrombocytopenia syndrome associated with viral vector COVID-19 vaccines. Eur J Intern Med. 2021. 89: 22-4
4. Cines DB, Bussel JB. SARS-CoV-2 vaccine-induced immune thrombotic thrombocytopenia. N Engl J Med. 2021. 384: 2254-6
5. Franchini M, Liumbruno GM, Pezzo M. COVID-19 vaccine-associated immune thrombosis and thrombocytopenia (VITT): Diagnostic and therapeutic recommendations for a new syndrome. Eur J Haematol. 2021. 107: 173-80
6. Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021. 384: 2092-101
7. Nazy I, Sachs UJ, Arnold DM, McKenzie SE, Choi P, Althaus K. Recommendations for the clinical and laboratory diagnosis of VITT against COVID-19: Communication from the ISTH SSC subcommittee on platelet immunology. J Thromb Haemost. 2021. 19: 1585-8
8. Oldenburg J, Klamroth R, Langer F, Albisetti M, von Auer C, Ay C. Diagnosis and management of vaccine-related thrombosis following AstraZeneca COVID-19 vaccination: Guidance statement from the GTH. Hamostaseologie. 2021. 41: 184-9
9. Pavord DSLester WMakris MScully MHunt B. Guidance from the Expert Haematology Panel (EHP) on COVID-19 Vaccine-Induced Immune Thrombocytopenia and Thrombosis (VITT). Available from: https://www.b-s-h.org.uk/media/19718/guidance-v20-20210528-002.pdf [Last accessed on 2021 Jul 17].
10. See I, Su JR, Lale A, Woo EJ, Guh AY, Shimabukuro TT. US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26 COV2. S vaccination, March 2 to April 21, 2021. JAMA. 2021. 325: 2448-56