- Department of Neurological Surgery, University of California San Francisco, San Francisco, California, United States.
Correspondence Address:
David J. Caldwell, Department of Neurological Surgery, University of California San Francisco, San Francisco, California, United States.
DOI:10.25259/SNI_105_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: David J. Caldwell, Justin K. Scheer, Gray Umbach, Manish K. Aghi. Acute hyponatremia post craniotomy resulting in a unilateral fixed and dilated pupil: A case study on diagnosis and management. 10-May-2024;15:160
How to cite this URL: David J. Caldwell, Justin K. Scheer, Gray Umbach, Manish K. Aghi. Acute hyponatremia post craniotomy resulting in a unilateral fixed and dilated pupil: A case study on diagnosis and management. 10-May-2024;15:160. Available from: https://surgicalneurologyint.com/surgicalint-articles/12886/
Abstract
Background: Postoperative hyponatremia is a known complication of intracranial surgery, which can present with depressed mental status. Hyponatremia resulting in focal neurologic deficits is less frequently described.
Case Description: We describe a patient who, after a bifrontal craniotomy for olfactory groove meningioma, developed acute hyponatremia overnight with a decline in mental status from Glasgow coma scale (GCS) score 15 to GCS 7 and a unilateral fixed dilated pupil. Head computed tomography showed expected postoperative changes without new acute or localizing findings, such as unilateral uncal herniation. The patient’s mental status and pupil immediately improved with the administration of mannitol; however, there was a subsequent decline in mental status with a preserved pupil later that morning. Hypertonic saline reversed the neurologic change, and the patient was eventually discharged without a neurologic deficit. Focal neurologic deficits need not always arise following a craniotomy from a postoperative hematoma, stroke, or other finding with radiographic correlate.
Conclusion: Post-craniotomy hyponatremia should now be seen as a postoperative complication that can result in both a general neurologic decline in mental status, as well as with focal neurologic signs such as a fixed, dilated pupil, which can be reversed with hyperosmolar therapy and correction of the hyponatremia.
Keywords: Craniotomy, Hyponatremia, Meningioma
INTRODUCTION
Intracranial surgery can result in a variety of postoperative complications, one of which is postoperative hyponatremia. This is a well-described phenomenon which often presents with depressed mental status and lethargy[
Postoperative hyponatremia can be due to several factors, which include water retention,[
CASE REPORT
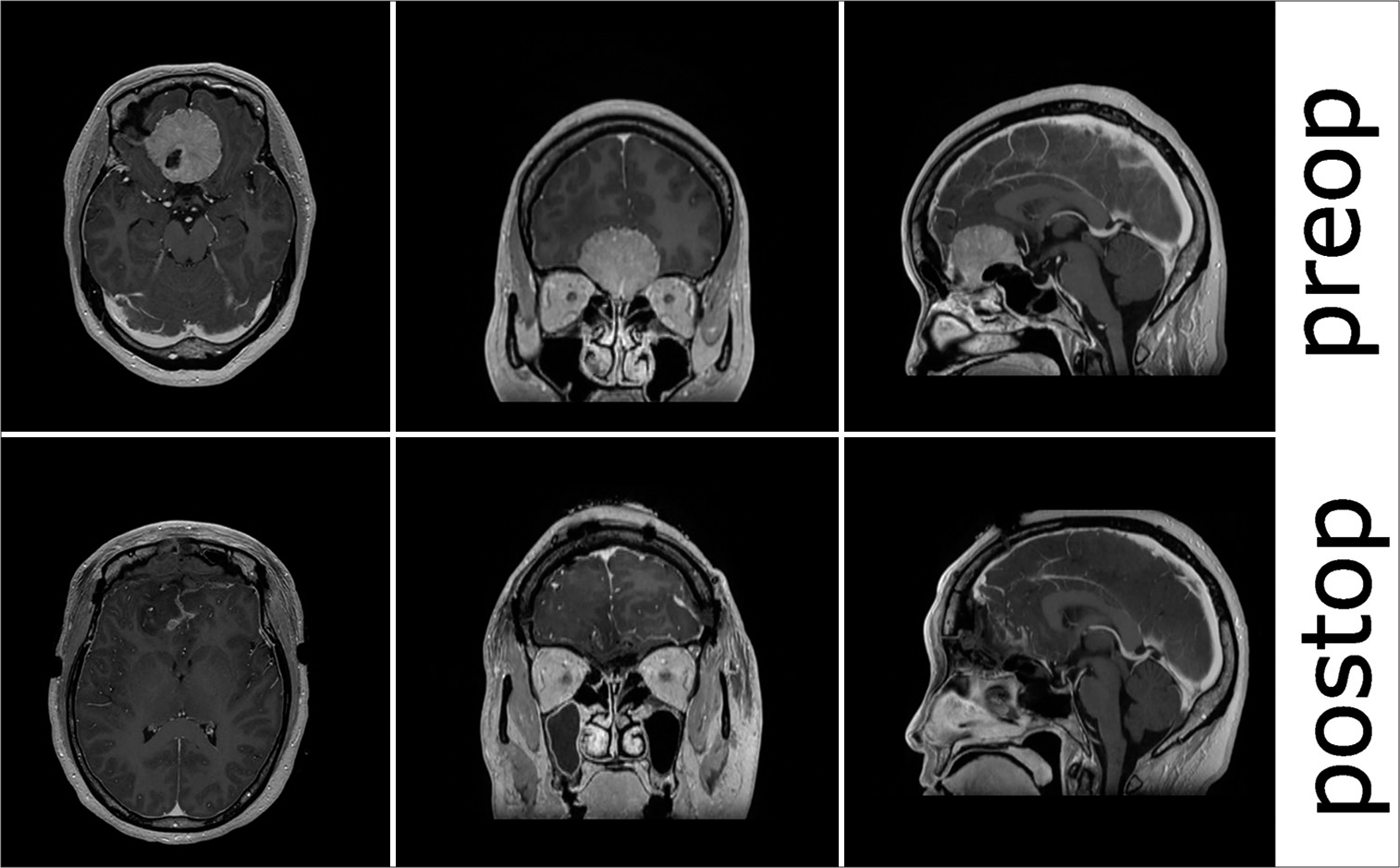
The patient was a 50-year-old female who presented initially with anosmia and headaches. Computed tomography (CT) and magnetic resonance imaging (MRI) revealed an enhancing extra-axial 4.1 × 4.3 × 3.6-centimeter mass originating from the cribriform plate with extension into the planum sphenoidale. She had no other focal neurologic deficits preoperatively. Her preoperative sodium was 138 millimoles/liter (mmol/L). On the day of surgery, she underwent an elective uncomplicated bifrontal craniotomy for tumor resection that took 4 h and 9 min. 63 g of mannitol was given intraoperatively. On her postoperative examination, she was alert and oriented ×3 but with perseverative speech. She was full strength on motor examination without any pronator drift and did not have any observable cranial nerve deficits. She was admitted to the neurologic intensive care unit (ICU) postoperatively with intravenous fluids (normal saline) running at 125 milliliters (mL)/hour (h). Her postoperative sodium was 139 mmol/L at 1 h postoperatively with a lactate of 5.1 mmol/L that was down-trending from a peak of 7.7 mmol intraoperatively. She had 5.1 liters (L) of total urine output (UOP) by 2 h postoperatively and an additional 2.5 L by 14 h postoperatively (an average of 206 mL/h for the 12 h in between). Her net fluid balance was −185 cc at the 14-h postoperative time point, and she had received 2 L of additional NS boluses overnight. Her evening sodium 7 h postoperatively was 132 mmol/L with preserved mental status. At this point, every 6-h sodium laboratory checks were begun, and urine studies were sent. Her urine specific gravity was 1.018, her urine osmolality was 698, and her urine sodium was 263. at this time, her serum sodium was 132, her serum creatinine 0.38, and her serum urea nitrogen 10. Her sodium decreased to 129 mmol/L 14 h postoperatively, and 3% was started at 30 mL/h. Shortly after, the patient had an acute decline in neurologic status after feeling nauseous and receiving prochlorperazine. On examination, her Glasgow coma scale (GCS) was GCS7 (E1V1M5), with localization in her bilateral upper extremities. Her right neurologic pupil index (NPI) was 0, with a size of 5.44 mm, and her left NPI was 3.2, with a size of 2.0 mm. She was protecting her airway. The decision was made to administer hyperosmolar therapy and obtain a CT due to the concern for a new mass lesion, such as postoperative hematoma causing her acute decline in GCS and a focal neurologic deficit. 25 g of mannitol was given at 15 h postoperatively, and her imaging was obtained shortly after [
Figure 1:
Pre- and post-operative day 1 computed tomography scans. The postoperative scan was acquired shortly after the patient developed a depressed neurologic status and focal neurologic deficit and minutes after mannitol had been started. Postoperative changes, but no focal mass lesions or postoperative complications were seen on the final read. Of note, the brain appears full both pre-and postoperatively.
DISCUSSION
Although previously known, acute hyponatremia can occur immediately postoperatively following a craniotomy, even without subarachnoid hemorrhage or disruption of the sellar region. The incidence of postoperative hyponatremia in the first 24 h appears rare, as a study looking at elective craniotomies for brain tumor surgeries looking at 188 patients demonstrated only one patient having diabetes insipidus and none having dysnatremia.[
Although hyponatremia is generally thought to result in an overall decline in mental status and lethargy, we show here an acute focal cranial nerve deficit without mass lesion on CT that reversed rapidly with correction of the hyponatremia using hyperosmolar therapy and sodium correction. Given an improvement in symptoms with the administration of mannitol, one possible mechanism of this change is cerebral edema secondary to hyponatremia that resulted in transient compression of the 3rd nerve; however, there was no imaging correlate seen. Of note, the patient was receiving mannitol before the CT scanner, so it is possible that there was a local mass effect that resolved by the time of imaging or was not well visualized on CT. A previous case study in a child with aneurysm subarachnoid hemorrhage demonstrated acute hyponatremia (Na 128) leading to a decline in GCS and evidence of transtentorial herniation on CT imaging, although the authors did not describe any focal cranial nerve deficit.[
The post-craniotomy neurologic decline can have several causes, including the mass effect (which can be secondary to a focal lesion such as a hematoma or, more in general, edema) and seizure. In one of the prior studies looking at 188 patients undergoing craniotomy, they reported ten patients who had a neurologic deterioration in the first 24 hours, with intracranial hematoma being found in 3.[
As for the origin of the hyponatremia, possible mechanisms for hyponatremia include SIADH and CSW. The patient had an elevated urine osmolality and urine sodium,[
As for the mechanism of hyponatremia causing brain edema, aquaporin 4 channels within astrocytes control water movement, and regulatory mechanisms in brain cells cause the immediate efflux of ions and, subsequently water to restore normal volume in the setting of hypotonicity.[
CONCLUSION
We describe a focal cranial nerve deficit acutely resulting after a craniotomy for a meningioma, most likely secondary to cerebral edema from craniotomy-related hyponatremia, with rapid recovery of neurologic function with hyperosmolar therapy and sodium correction. No imaging correlate explained the focal deficit. These findings are limited by the number of subjects (n = 1), as well as the fact that our postoperative imaging during the episode of hyponatremia was limited to a CT, restricting our ability to assess for specific MRI findings such as edema better seen on a T2 sequence, cranial nerves, or the extent of residual tumor. Despite our limitations, these findings are relevant broadly to cranial neurosurgery, as sodium imbalance is a common postoperative problem and can have significant morbidity and mortality if not identified and managed. Not all focal neurologic signs need to be secondary to a postoperative hemorrhage or a mass lesion and may be a sign of worsened cerebral edema secondary to electrolyte disturbances requiring swift correction.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Berendes E, Walter M, Cullen P, Prien T, Aken HV, Horsthemke J. Secretion of brain natriuretic peptide in patients with aneurysmal subarachnoid haemorrhage. Lancet. 1997. 349: 245-9
2. Cabantog AM, Bernstein M. Complications of first craniotomy for intra-axial brain tumour. Can J Neurol Sci. 1994. 21: 213-8
3. Carpenter J, Weinstein S, Myseros J, Vezina G, Bell MJ. Inadvertent hyponatremia leading to acute cerebral edema and early evidence of herniation. Neurocrit Care. 2007. 6: 195-9
4. Danziger J, Zeidel ML. Osmotic homeostasis. Clin J Am Soc Nephrol. 2015. 10: 852-62
5. Elsamadicy AA, Sergesketter A, Adogwa O, Ongele M, Gottfried ON. Complications and 30-day readmission rates after craniotomy/craniectomy: A single Institutional study of 243 consecutive patients. J Clin Neurosci. 2018. 47: 178-82
6. Fox JL, Falik JL, Shalhoub RJ. Neurosurgical hyponatremia: The role of inappropriate antidiuresis. J Neurosurg. 1971. 34: 506-14
7. Harrigan MR. Cerebral salt wasting syndrome. Crit Care Clin. 2001. 17: 125-38
8. Hassan ZU, Kruer JJ, Fuhrman TM. Electrolyte changes during craniotomy caused by administration of hypertonic mannitol. J Clin Anesth. 2007. 19: 307-9
9. Lonjaret L, Guyonnet M, Berard E, Vironneau M, Peres F, Sacrista S. Postoperative complications after craniotomy for brain tumor surgery. Anaesth Crit Care Pain Med. 2017. 36: 213-8
10. Madden JR, Dobyns E, Handler M, Foreman NK. Experience with electrolyte levels after craniotomy for pediatric brain tumors. J Pediatr Oncol Nurs. 2010. 27: 21-3
11. Michinaga S, Koyama Y. Pathogenesis of brain edema and investigation into anti-edema drugs. Int J Mol Sci. 2015. 16: 9949-75
12. Nelson PB, Seif SM, Maroon JC, Robinson AG. Hyponatremia in intracranial disease: Perhaps not the syndrome of inappropriate secretion of antidiuretic hormone (SIADH). J Neurosurg. 1981. 55: 938-41
13. Palmer BF. Hyponatraemia in a neurosurgical patient: Syndrome of inappropriate antidiuretic hormone secretion versus cerebral salt wasting. Nephrol Dial Transplant. 2000. 15: 262-8
14. Unterberg AW, Stover J, Kress B, Kiening KL. Edema and brain trauma. Neuroscience. 2004. 129: 1019-27
15. Varaldo E, Berton AM, Maccario M, Gasco V. Isolated third cranial nerve palsy associated with sudden worsening of hypotonic hyponatremia secondary to ischemic pituitary apoplexy. Endocrines. 2023. 4: 664-71
16. Wise BL. Fluid and electrolyte balance following craniotomy. J Neurosurg. 1956. 13: 223-34
17. Wise BL. Hyponatremia following craniotomy. AMA Arch Neurol. 1960. 2: 391-8