- Department of Neurosciences, Neurosurgery Unit, AORN Santobono-Pausilipon Children’s Hospital, Naples, Italy.
- Department of Neurosciences and Reproductive and Dental Sciences, Division of Neurosurgery, Federico II University of Naples, Naples, Italy.
- Department of Neurosciences, Neuroradiology Unit, AORN Santobono-Pausilipon Children’s Hospital, Naples, Italy.
Correspondence Address:
Pietro Spennato, Department of Neurosurgery, AORN Santobono-Pausillipon, Naples, Italy.
DOI:10.25259/SNI_434_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Francesca Vitulli1,2, Pietro Spennato1, Domenico Cicala3, Giuseppe Mirone1, Maria Rosaria Scala1,2, Giuseppe Cinalli1. Acute ischemic stroke secondary to ventriculoperitoneal shunt dysfunction in a child with Moyamoya syndrome. 15-Jul-2022;13:306
How to cite this URL: Francesca Vitulli1,2, Pietro Spennato1, Domenico Cicala3, Giuseppe Mirone1, Maria Rosaria Scala1,2, Giuseppe Cinalli1. Acute ischemic stroke secondary to ventriculoperitoneal shunt dysfunction in a child with Moyamoya syndrome. 15-Jul-2022;13:306. Available from: https://surgicalneurologyint.com/surgicalint-articles/11718/
Abstract
Background: Patients with brain vascular disease and hydrocephalus may be predisposed to acute ischemic stroke in case of shunt dysfunction and subsequent increased intracranial pression. Patients with brain tumor may develop hydrocephalus as a consequence of obstruction of cerebrospinal fluid pathways and radiation-induced moyamoya syndrome secondary (RIMS) to radiotherapy (RT).
Case Description: A 15-year-old male patient, affected by hydrocephalus and RIMS, presented acute cerebral ischemia after an episode of shunt malfunction. The shunt was promptly revised and the areas of ischemia visible at magnetic resonance imaging significantly decreased.
Conclusion: Children who receive RT for brain tumor, particularly if the circle of Willis region is involved, require close surveillance for the development of vasculopathy and consequent stroke. This surveillance must be even tighter if the patient has been treated with ventricular shunt for the possible synergistic interaction between the two causes on reducing cerebral perfusion and increasing the risk of acute ischemic events.
Keywords: Radiation-induced moyamoya syndrome, Transient ischemic attack, Ventriculoperitoneal shunt
INTRODUCTION
Coexistence of brain vascular disease and hydrocephalus treated with ventriculoperitoneal shunt may predispose to acute ischemic stroke in case of shunt dysfunction and subsequent increased intracranial pressure (ICP). Increased ICP, further, decreases cerebral perfusion pressure (CPP) that is already impaired in patients with vasculopathy. This is a common phenomenon in patients with critical disease (traumatic brain injury and subarachnoid hemorrhage) and lost of vascular autoregulation.
The association of vasculopathy and hydrocephalus is not so uncommon: patients with suprasellar tumor treated by surgery and adjuvant radiotherapy (RT) may develop hydrocephalus as a consequence of obstruction of cerebrospinal fluid (CSF) pathways, and radiation-induced moyamoya syndrome (RIMS) secondary to RT.[
Surprisingly, to the best of our knowledge, no cases of acute ischemic stroke at time of shunt dysfunction in patient with cerebral vasculopathy have been reported in the literature.
We, herein, report a case of a 15-year-old male patient with a history of hydrocephalus secondary to optic pathway gliomas (OPG) and RIMS who developed acute cerebral ischemia caused by shunt dysfunction, with secondary partial resolution of ischemic areas after normalization of ICP.
CASE DESCRIPTION
This patient, at the age of 1 year, had a diagnosis of pilocytic astrocytoma of optic pathway that was initially treated with surgical debulking and chemotherapy (CT), according to SIOP 2004 LGG protocol. Due to tumor progression, over the next 3 years, he underwent several surgical procedures and different CT regimens (Carboplatin/ Vincristine; Cisplatinum/Vincristine/Cyclophosphamide; and Bevacizumab/Irinotecan). He also developed a hydrocephalus treated with ventriculoatrial shunt at the age of 1 year transformed in ventriculoperitoneal shunt after 6 years. The tumor progressed under chemo so at age of 4 he underwent surgical debulking followed by fractionated RT with a total dose of 54 Gy. One year after RT, he presented multiple episodes of transient ischemic attacks with the right facial palsy and aphasia. Magnetic resonance imaging (MRI), MRI angiography, computed tomography angiography, and digital subtraction arteriography highlighted a tight stenosis of the supraclinoidal internal carotid artery (ICA), M1, and A1 segments on the left side, with a contralateral hypoplastic A1 [
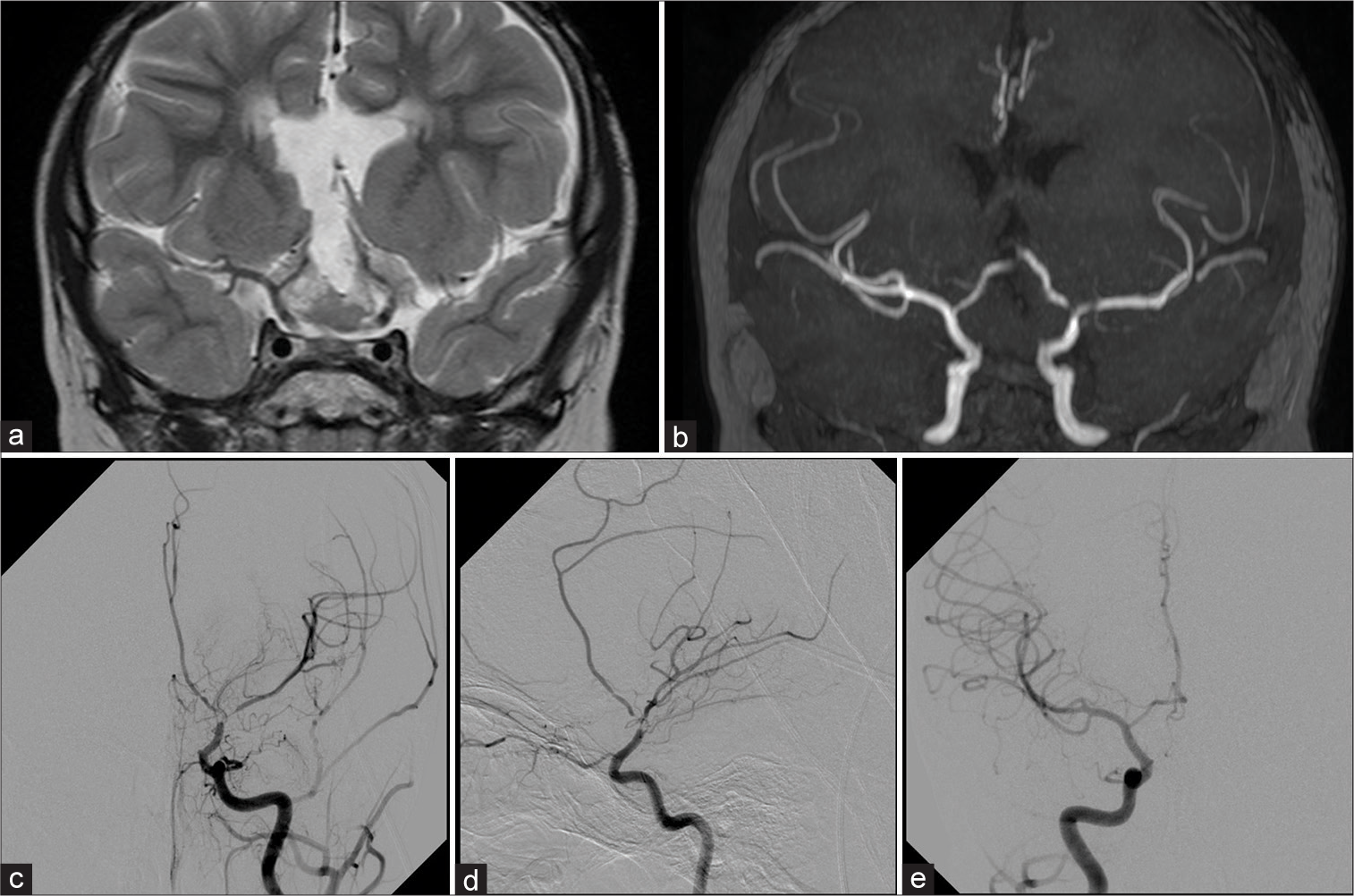
Figure 1:
Coronal TSE T2-weighted image (a) shows the residual tumor mass that enchases the terminal ICAs. The coronal MIP reconstruction of TOF MR Angiography (b) shows lack of signal in the terminal part of the left ICA due to severe stenosis and slight reduction of signal in the distal MCA branches. The left ICA angiograms on frontal (c) and lateral (d) projections confirm the severe stenosis of the terminal part of the ICA, proximal M1, and A1 segments; slight evidence of basal moyamoya network is also observed. No significant narrowing is observed on the right ICA frontal angiogram (e).
At the age of 15, he was readmitted with a 4-day history of headache and progressive depressed level of consciousness. At admission, clinical condition quickly worsened and he was transferred in intensive care unit with a Glasgow Coma Scale 3/15 and mydriatic pupils. MRI showed increment of ventricular size with restricted diffusion areas on diffusion-weighted images of distal territory of ICAs and left middle cerebral artery (MCA), compatible with acute ischemic injury [
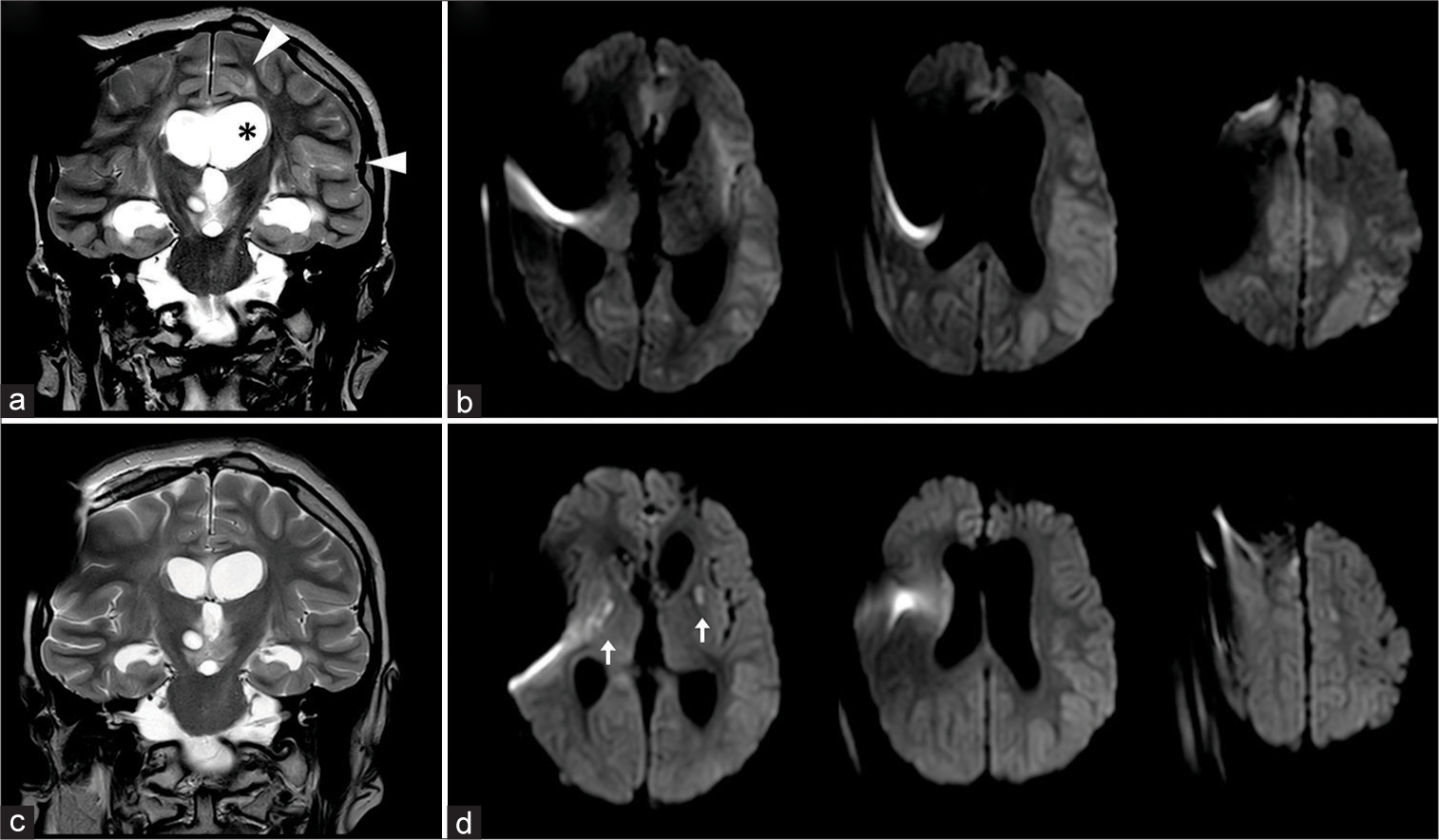
Figure 2:
Coronal TSE T2-weighted (a) and axial b-1000 DWI images (b) in acute phase of shunt dysfunction show dilated ventricles (asterix in 2a) and acute ischemic changes on MCA and ACA cortical territories, with restricted diffusion, effaced sulci and scissures and cortical edema (arrowheads in 2a). Early postoperative images (c and d) show progressive improvement of cortical ischemic areas; new-onset deep ischemic lesions in basal ganglia are also observed (arrows).
The patients had a slow recovery until the restoration of his usual clinical conditions after 3 months of rehabilitation.
DISCUSSION
RT is an effective therapeutic modality for the treatment of many pediatric brain tumors, playing a role in local disease control and improving patients’ overall survival, especially those patients who do not respond to CT.[
RIMS develops particularly in patients with tumors in close proximity to the circle of Willis.[
Pathogenesis of RIMS is debated in the literature: several authors support the theory of a chronic arteritis obliterans caused by the formation of reactive oxygen species that provoke reactive proliferation of the endothelial lining and subendothelial connective tissue with progressive stenosis of vascular lumen.[
The major aspect of RIMS is the total/partial absence of collateral circulation as compared with traditional Moyamoya disease.[
Although RT is the major risk factor for later cerebrovascular events, another mechanism that may contribute to the onset of cerebral ischemia is the decrease of CPP with compromised brain oxygenation caused by the raise of ICP.[
The CBF is regulated by the Monroe-Kellie doctrine[
CONCLUSION
Children receive RT for brain tumor, particularly if the circle of Willis region is involved, require close surveillance for the development of vasculopathy and consequent stroke. In our opinion, this surveillance must be even tighter if the patient has been treated with ventricular shunt for the possible synergistic interaction between the two causes on CBF and the risk of acute ischemic events. In particular, symptoms of shunt malfunction should be promptly recognized, and adequate treatment configured.
Statement of ethics
Written informed consent was obtained from the parents of the patient for publication of this case report and any accompanying images. All identifying information were stripped off. The manuscript was conducted ethically in accordance with the World Medical Association Declaration of Helsinki.
Author contributions
Conception of the work: FV, PS; Acquisition of data for the work: FV, DC; Drafting the work: FV, PS; Revising it critically for important intellectual content: GM, MRS; Final approval of the version: GC.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Campen CJ, Kranick SM, Kasner SE, Kessler SK, Zimmerman RA, Lustig R. Cranial irradiation increases risk of stroke in pediatric brain tumor survivors. Stroke. 2012. 43: 3035-40
2. Currie S, Raghavan A, Batty R, Connolly DJ, Griffiths PD. Childhood moyamoya disease and moyamoya syndrome: A pictorial review. Pediatr Neurol. 2011. 44: 401-13
3. Desai SS, Paulino AC, Mai WY, Teh BS. Radiation-induced moyamoya syndrome. Int J Radiat Oncol Biol Phys. 2006. 65: 1222-7
4. Figaji AA, Zwane E, Fieggen AG, Argent AC, Le Roux PD, Siesjo P. Pressure autoregulation, intracranial pressure, and brain tissue oxygenation in children with severe traumatic brain injury. J Neurosurg Pediatr. 2009. 4: 420-8
5. Gorbunov NV, Kiang JG. Brain damage and patterns of neurovascular disorder after ionizing irradiation. Complications radiotherapy and radiation combined injury. Radiat Res. 2021. 196: 1-16
6. Kim TG, Kim DS, Chung SS, Choi JU. Moyamoya syndrome after radiation therapy: Case reports. Pediatr Neurosurg. 2011. 47: 138-42
7. Mirone G, Cicala D, Meucci C, D’Amico A, Santoro C, Muto M. Multiple burr-hole surgery for the treatment of moyamoya disease and quasi-moyamoya disease in children: Preliminary surgical and imaging results. World Neurosurg. 2019. 127: e843-55
8. Monro A.editors. Observations on the Structure and Functions of the Nervous System. Edinburgh: Creech, Johnson; 1783. p.
9. Passos J, Nzwalo H, Marques J, Azevedo A, Netto E, Nunes S. Late cerebrovascular complications after radiotherapy for childhood primary central nervous system tumors. Pediatr Neurol. 2015. 53: 211-5
10. Rohlwink UK, Zwane E, Fieggen AG, Argent AC, Le Roux PD, Figaji AA. The relationship between intracranial pressure and brain oxygenation in children with severe traumatic brain injury. Neurosurgery. 2012. 70: 1220-30
11. Scala M, Fiaschi P, Cama A, Consales A, Piatelli G, Giannelli F. Radiation-induced moyamoya syndrome in children with brain tumors: Case series and literature review. World Neurosurg. 2020. 135: 118-29
12. Ullrich NJ, Robertson R, Kinnamon DD, Scott RM, Kieran MW, Turner CD. Moyamoya following cranial irradiation for primary brain tumors in children. Neurology. 2007. 68: 932-8
13. Wang C, Roberts KB, Bindra RS, Chiang VL, Yu JB. Delayed cerebral vasculopathy following cranial radiation therapy for pediatric tumors. Pediatr Neurol. 2014. 50: 549-56