- Theoretical Neuroscience Research, LLC, Ridgeland, Mississippi, United States.
Correspondence Address:
Russell L. Blaylock, Retired Neurosurgeon, Theoretical Neuroscience Research, LLC, Ridgeland, Mississippi, United States.
DOI:10.25259/SNI_296_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Russell L. Blaylock. Additive aluminum as a cause of induced immunoexcitoxicity resulting in neurodevelopmental and neurodegenerative disorders: A biochemical, pathophysiological, and pharmacological analysis. 24-May-2024;15:171
How to cite this URL: Russell L. Blaylock. Additive aluminum as a cause of induced immunoexcitoxicity resulting in neurodevelopmental and neurodegenerative disorders: A biochemical, pathophysiological, and pharmacological analysis. 24-May-2024;15:171. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=12907
Abstract
Much has been learned about the neurotoxicity of aluminum over the past several decades in terms of its ability to disrupt cellular function, result in slow accumulation, and the difficulty of its removal from cells. Newer evidence suggests a central pathophysiological mechanism may be responsible for much of the toxicity of aluminum and aluminofluoride compounds on the brain and spinal cord. This mechanism involves activation of the brain’s innate immune system, primarily the microglia, astrocytes, and macrophages, with a release of neurotoxic concentrations of excitotoxins and proinflammatory cytokines, chemokines, and immune mediators. Many studies suggest that excitotoxicity plays a significant role in the neurotoxic action of several metals, including aluminum. Recently, researchers have found that while most of the chronic pathology involved in the observed neurodegenerative effects of these metals are secondary to prolonged inflammation, it is the enhancement of excitotoxicity by the immune mediators that are responsible for most of the metal’s toxicity. This enhancement occurs through a crosstalk between cytokines and glutamate-related mechanisms. The author coined the name immunoexcitotoxicity to describe this process. This paper reviews the evidence linking immunoexcitotoxicity to aluminum’s neurotoxic effects and that a slow accumulation of aluminum may be the cause of neurodevelopmental defects as well as neurodegeneration in the adult.
Keywords: Accumulation in neurons and glia, Aluminofluoride complex, Aluminum, Excitotoxicity, Immunoexcitotoxicity, Microglial activation, Nanoscaled aluminum, Neurodegeneration, Sickness behavior
INTRODUCTION
While aluminum is the third most common metal found in the earth’s crust, it has no role to play in normal human physiology, biochemistry, or maintaining health.[
BASIS OF ALUMINUM IMMUNOEXCITOXICITY
While aluminum is not recognized as a redox metal, it can induce significant inflammation within various tissues. Redox refers to a variation in the valence of a metal that induces a greater risk of free radical generation, such as with ferric (Fe+3) and ferrous (Fe+2) iron interchange. In chemistry, the loss of electrons makes the metal a free radial, and an excess of electrons makes it an electron donor or antioxidant. Exley explained that while aluminum is a non-redox metal, under certain conditions, it can act as a pro-oxidant.[
Aluminum may interfere with the normal biochemistry of cells by a number of mechanisms. One interesting to chronic brain inflammation is the finding that aluminum produces a profound general decrease in nicotine binding involving all brain areas.[
Another way in which aluminum may contribute to neuronal injury, particularly associated with AD, is by interference with calcium homeostasis, which is known to be perturbed in AD and other neurodegenerative disorders.[
Figure 1:
Synaptic illustration showing trafficking of AMPAR initiated by activation of tumor necrosis factor (TNF) R1 by high levels of TNF-alpha, which then releases GluR 2-lacking AMPA receptors from the endoplasmic reticulum. This AMPA type of receptor allows calcium entry into the neuron, thus making it much stronger and potentially more destructive. Internalization of the gamma-aminobutyric acid inhibitory receptor is not shown but occurs when stimulated by inflammation. Na+: Sodium, Ca2+: Calcium, AMPA:α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid, NMDA:N-methyl-D-aspartate,Mg 2+: Magnesium, TNFR: Tumor necrosis factor receptor, AMPA: α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid, MAPK: Mitogen-activated protein kinase, CREB: cAMP-response element binding protein, CaMK:Calmodulin-dependent protein kinase, PKC: Protein kinase C.
By activating the inflammatory cytokines, especially tumor necrosis factor-alpha (TNF-α), one sees events that can enhance excitotoxicity; in this case, the GABA receptors (an inhibitory neurotransmitter-controlled receptor) are trafficked inside the neuron, thus enhancing excitotoxicity. Other mechanisms also link inflammatory stimulation to excitotoxicity (immunoexcitotoxicity) [
In another study, Campbell et al. observed brain inflammation in animals exposed to aluminum lactate when added to their drinking water.[
It is known that metabotropic glutamate receptor (mGLuR) signaling, as well as that of many other neurotransmitters, is critically dependent on G-protein receptor systems.[
By interfering with normal mGLuR function, aluminum and especially aluminofluoride complexes could potentially cause malfunctioning of important brain pathways as well as abnormal architectonic development of the brain.[
Aluminum can also enhance excitotoxicity by inducing apoptosis of astrocytes, which are thought to be a primary site of aluminum accumulation.[
Aluminum is also known to operate synergistically with other toxic metals, such as copper and iron, to increase brain and spinal cord inflammation.[
The inflammatory cytokines are known to act on several enzymes and mechanisms to enhance excitotoxicity.[
This is especially true of TNF-α. TNF-α, which is elevated with aluminum exposure [
These observations indicate a mechanism by which aluminum can induce immunoexcitotoxicity, a process based on deleterious interactions between inflammatory cytokines (i.e., TNF-α) and excitotoxins (i.e., glutamate). For example, Matyja demonstrated that exposing organotypic cultures of rat hippocampus for 24 h to a combination of aluminum and glutamate, both in subtoxic concentrations, produced typical excitotoxic lesions, which predominantly consisted of mitochondrial abnormalities, both structural and biochemical.[
It has also been shown that aluminum, in combination with other metals (copper and iron), additively increases brain inflammation.[
It is important to know what proportion of absorbed aluminum reaches the brain in comparison to other organs. To answer this question, Flarend et al. injected New Zealand White rabbits intramuscularly with 26Al radiolabeled aluminum hydroxide and aluminum phosphate and traced their distribution at 28 days post-injection in blood and urine samples as well as tissues by utilizing accelerator mass spectrometry.[
It is of significant concern that low levels alone of environmental aluminum are sufficient to induce neurotoxic outcomes.[
We have a combination of in vitro and in vivo studies that provide indisputable evidence that aluminum can significantly increase the level of proinflammatory cytokines and glutamate (and other excitotoxins) in the CNS.[
ALUMINUM AND MITOCHONDRIAL ENERGY PRODUCTION
Aluminum has been shown to interfere with the action of membrane receptors (i.e., G-protein coupled receptors [GPCRs]) cell signaling pathways. It also alters deoxyribonucleic acid integrity and impairs mitochondrial function, all of which will have an enhancing effect on both excitotoxicity in general and, specifically, immunoexcitotoxicity. [
Aluminum is known to accumulate in the mitochondria and disrupt several mitochondrial functions, leading to an energy deficit,[
Aluminum is known to concentrate in the mitochondria of neurons, microglia, and astrocytes and reduces energy production.[
Mitochondria are also responsible for calcium regulation within the cell.[
Several toxic metals, industrial chemicals, solvents, and some pesticides are associated with mitochondrial dysfunction.[
Of particular importance is the effect of aluminum on cell signaling pathways, such as G-proteins, phosphatidylinositol-specific phospholipase C, protein kinase C, and calcium homeostasis.[
One should also avoid glutamate, either as added during processing or naturally present, as it is in certain foods. This would include most nuts, beans (especially black beans), red meats, chicken, cheeses, and mushrooms as natural sources of higher glutamate levels. Processes food have very high levels under names either as monosodium glutamate or disguised names, such as hydrolyzed soy, hydrolyzed proteins, natural flavors, autolytic enzymes, protein concentrates, and an evolving number of new names. As stated, this not only increases aluminum levels in the nervous system but also renders the glutamate much more destructive and, in addition, raises brain glutamate levels.[
Other aluminum forms, such as aluminosilicates used in tap water, have also been reported in diseased brains (i.e., in the cores of senile plaques in AD patients).[
Some of the highest levels of aluminum are found in black tea, and studies have shown that the longer this is brewed, the higher the aluminum level. Tea should not be brewed over 3 min. White tea, the youngest to be harvested, has the lowest aluminum levels. Tea grown in India has been shown to have the lowest aluminum levels, especially for white tea. Chinese tea has a much higher level. Green teas are intermediate in aluminum levels, also with the lowest level in green tea grown in India. Black tea, high in aluminum, is associated with neurodegenerative diseases. Coffee does not contain high aluminum levels.
Taken together, all the above experimental observations indicate that by promoting oxidative damage and inflammation in the CNS, exacerbating excitotoxic damage by (i) increasing the levels of excitotoxic mediators and (ii) impeding their clearance, aluminum can both trigger and promote neuronal injury. Aluminum-induced microglial/astrocyte-mediated immunoexcitotoxicity combined with its direct neurotoxic effects makes this element a strong candidate for at least enhancing neurodegeneration associated with such disorders as AD, PD, ALS, HD, MS, viral encephalopathies, and chronic traumatic encephalopathy. Notably, all these diseases have been previously linked to over-active glia.[
The excitotoxic cascade can be triggered by an excessive release of glutamate from microglia and/or astrocytes, with elevations in nitric oxide (NO), proinflammatory prostanoids, and generation of a number of ROS/RNS.[
The largest aluminum exposure from vaccines occurs during initial vaccinations soon after birth and during early childhood. Should a child follow the recommended vaccine schedule for the United States, they will receive a total of 5 mg of aluminum by 2 years of age from a total of 17 aluminum-adjuvanted pediatric vaccines.[
AN INTEGRATED HYPOTHESIS OF IMMUNOEXCITOTOXICITY LINKING ALL OF THIS DATA
The mechanism by which systemic activation of brain microglia occurs is critical to understanding the effect of sequential immune stimulation with immune adjuvants, including aluminum. When microglia are first exposed to a disturbance in homeostasis, they may assume a primed state in which their messenger RNA (mRNA) and membrane receptors are upregulated, but there is no increased release of cytokines, chemokines, interferons or excitotoxins.[
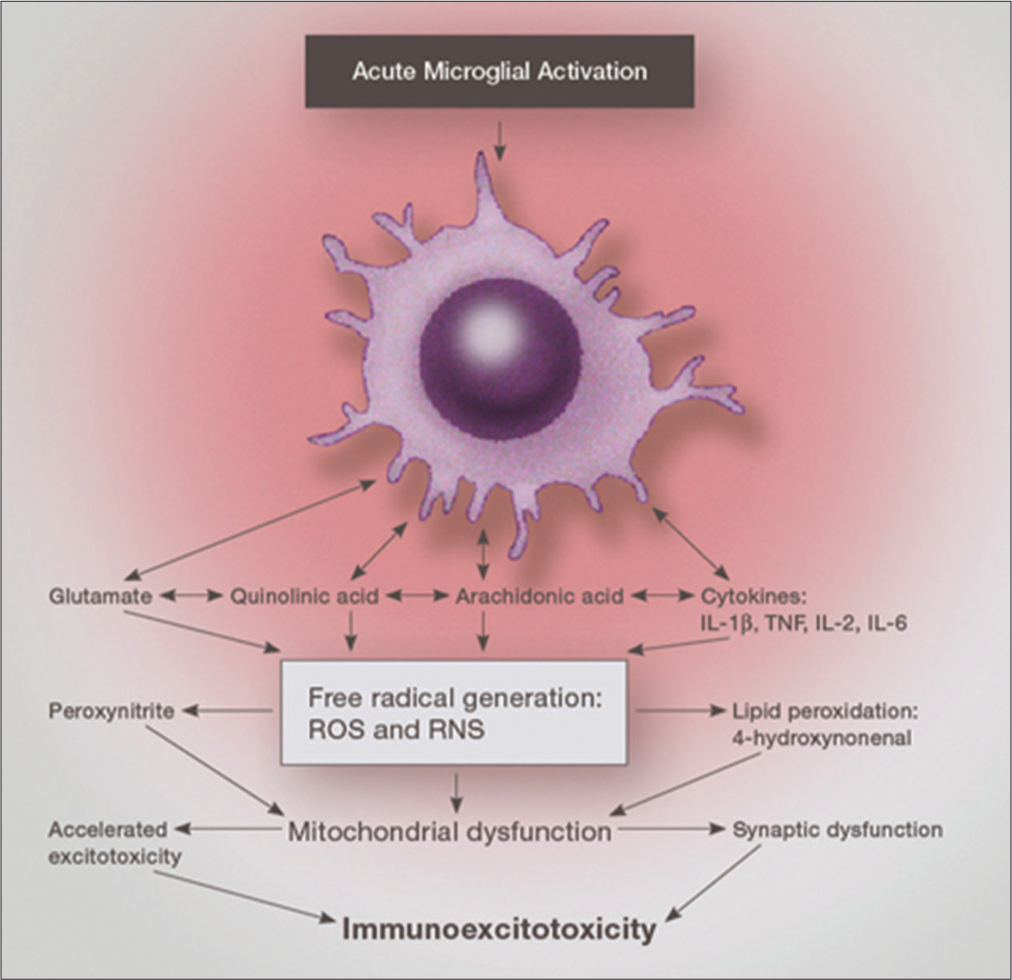
Subsequent stimulation will activate these primed microglia with a hyper-responsive reaction occurring, leading to several-fold higher concentrations of released proinflammatory cytokines, chemokines and the release of three excitotoxins – glutamate, aspartate, and quinolinic acid (QUIN).[
The pathophysiological point of initiation of histological destruction by these toxic substances entails the immune system as the starting point. Involved in immunoexcitotoxicity is the brain’s innate immune system, primarily involving microglia, macrophages, and astrocytes. Microglia make up 5–15% of the cells in the CNS cortical grey matter, hippocampus, olfactory telencephalon, and basal ganglion.[
The purpose of this review was to describe two mechanisms linking aluminum to neurodegenerative processes and neurological dysfunction – (1) systemic activation of CNS microglia and (2) resulting immunoexcitotoxicity. The first of these mechanisms, referred to as sickness behavior, has been extensively studied and provides both a direct and indirect link between systemic immune activation, activation of CNS microglia, and abnormal neurological symptoms.[
The surface of microglia contains a number of receptors, including receptors for most of the neurotransmitters, pro- and anti-inflammatory cytokines, chemokines, interferons, and major histocompatibility complex Classes I and II receptors.[
There is also evidence that systemically administered aluminum from drinking water can specifically activate TNF-α without activating other cytokines. For example, Tsunoda and Sharma exposed male BALB/c mice to aluminum sulfate-containing drinking water ad libitum at concentrations of 0, 5, 25, and 125 ppm aluminum for 1 month and found significant expression of TNF-α mRNA in the cerebral cortex.[
There is growing evidence that both glutamate and immune cytokines play crucial roles in various aspects of brain development.[
There is suggestive evidence that microglia can become stuck in a neurodestructive mode over long periods.[
Two forms of aluminum are of special concern: Aluminum-L-glutamate and nanoscaled aluminum, both of which have high absorption from the gut and passage into the brain, as well as higher toxicity profiles than aluminum alone. Adding to this concern is the fact that glutamate, both as a food additive and naturally occurring in foods, is common in the Western diet. Deloncle et al. injected Al-L-glutamate subcutaneously and i.v. for 5 weeks and demonstrated a significant increase in aluminum content in several areas of the animal’s brain, including the hippocampus, occipitoparietal cortex, cerebellum, and striatum.[
We must keep in mind that when it rains, the nano aluminum sprayed in the air is deposited in the groundwater, lakes, rivers, and streams. As a result, all plants will now have a higher aluminum level.
When assessing aluminum’s potential toxicity, one must also consider absorption and distribution. While absorption from foods is considered to be quite small, under certain conditions, this can be increased substantially. For example, organic acids and some amino acids increase aluminum absorption significantly. Increasing absorption is as follows: Aluminum citrate > aluminum tartrate > aluminum gluconate > aluminum lactate > aluminum glutamate > aluminum chloride, aluminium sulfate, and aluminum nitrate.[
When considering immunoexcitotoxicity, one is concerned with two events regulated by activated microglia: (i) proinflammatory cytokine/chemokine release and (ii) release of excitatory amino acids, particularly glutamate, quinolinic acid and aspartate – all excitotoxins. It is the excitotoxicity component rather than inflammation alone that appears to be the main pathological mechanism for actual damage to neurons and their processes. For example, research shows that even exposure to high concentrations of TNF-alone for 24 hours a day for 6 days does not result in neuronal death.[
Of particular interest is the observation by SuarezFernandez et al. that chronic aluminum exposure in mixed cultures of astrocytes and neurons results in significant astroglial apoptosis and associated neuronal loss.[
NATURAL PLANT EXTRACTS THAT REMOVE AND/OR NEUTRALIZE ALUMINUM TOXICITY IN CELLS
Fortunately, there are several natural products that can either chelate and remove aluminum from biological systems or render it non-toxic without its removal. One of the most impressive is curcumin.[
Curcumin can improve the mechanisms within the mitochondria, such as improved biogenesis and fusion, reduce the accumulation of beta-amyloid toxicity, reduce its inflammatory effects, and, thereby, in the process, improve synaptic activity and its functional proteins.[
Naringin, a plant flavonoid, has been shown to prevent memory impairment and mitochondrial oxidative damage in aluminium-exposed rats.[
It has also been shown that the probiotic Lactobacillus plantarum CCFM639 also significantly protects multiple organs, including the brain, from damage by aluminum and lowered significantly aluminum levels.[
A SLOW ACCUMULATION OF ALUMINUM OVER A LIFETIME
Most people, even many physicians, assume that toxins are dangerous in high acute doses. As a result, many ignore the aspect of a slow progressive accumulation in tissues and cells. We now know that aluminum accumulates slowly and that low levels can eventually reach toxic levels.[
Of special concern are injections of vaccines containing aluminum adjuvants, as 100% of this aluminum is absorbed, unlike that taken orally. The toxicity also depends on the person’s size and weight. Infants and small children are much more vulnerable than adults based on these considerations.[
Yet, even in adults, one can gradually accumulate enough aluminum in the CNS, its neurons and glia to eventually result in neurodegeneration as one ages. Aluminum appears to be very difficult to remove from the cells and can persist and gradually accumulate at high enough levels to result in neurodegeneration and CNS disease.[
It has been noted that more younger people are getting ALS than in the past, which was at that time considered a disease of the older person. It has been shown that the aluminum burden from the present vaccine schedule for youths, including babies as young as 6 months of age, has reached levels far higher than that deemed safe for adult oral consumption, which is only partially absorbed. It has been shown that systemic macrophages rapidly consume injected aluminum from the adjuvants and is carried to the CNS (Macrophages are very difficult to distinguish from native microglia and have similar functions in the CNS).[
While the vaccine schedule has now been expanded so that the average child will receive over 40 injections before starting school, which means the child is getting a very high dose of aluminum rather rapidly, as the accumulative dose is the sum of the individually added adjuvants. A significant amount of this aluminum is stored in the brain and spinal cord glia and neurons. Many pediatricians will give as many as 6–9 injections on a single office visit.
With adults getting yearly influenza vaccines and a number of other vaccines, such as the hepatitis B vaccine, tetanus vaccines, the shingle vaccine, and the pneumococcal vaccines, to say nothing of the very high level of aluminum in the Gardasil vaccines, one is not surprised that we are seeing the high degree of illness and especially neurodegenerative disease, in our population.[
What concerns me is that these children are receiving a dose of neurotoxic aluminum that is also being deposited in their spinal cord, especially the motor cells. These cells have a high energy requirement and are thus susceptible to being highly sensitive to excitotoxicity triggered by the aluminum in the neuron as well as the surrounding glia.
Dr. Chris Shaw is the head of neuroscience in Vancouver and an expert in research concerned with autism spectrum disorders and ALS. I asked him how often he saw microglial activation in his studies on aluminum and ALS. He responded, “Always.”[
With people getting an influenza injection each year, as well as other highly recommended vaccines, we are also seeing a significant rise in all neurodegenerative diseases over the past decade. To most of the medical profession, this is a mystery. We are also seeing a rise in the use of pesticides and herbicides, which, in addition, make a major contribution to the rise in neurodegenerative diseases. We must think in terms of toxin synergy.
CONCLUSION
Aluminum is a major neurotoxin mainly triggering immunoexcitoxicity. During a person’s lifetime, the concentration of aluminum slowly builds from oral absorption from food stuffs [incomplete], inhalation from the atmosphere, and drinking tap water. In addition, injected aluminum as found in many vaccine adjuvants in the childhood vaccine schedule contain aluminum that is 100% absorbed. The early exposure to this toxic metal can also interfere with the proper neurodevelopment of the nervous system, both by activating the inflammatory pathways and excitotoxicity chronically [immunoexcitotoxicity] during this critical period that is rather intense over the first several years of life.
As a person ages, not only are the neurons and glia undergoing pathophysiological assaults by aluminum, both from such vaccine adjuvants and that absorbed from food and water, which accumulate over a lifetime, resulting in sensitive cells and subcellular organelles receiving a pathophysiological dose within these critical neural cells and structures.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent was not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Aremu DA, Meshitsuka S. Some aspects of astroglial functions and aluminum implications for neurodegeneration. Brain Res Rev. 2006. 52: 193-200
2. Arun S, Liu L, Donmez G. Mitochondrial biology and neurological diseases. Curr Neuropharmacol. 2016. 14: 143-54
3. Assmann CE, Mostardeuro VB, Weis GC, Reichert KP, Alves AO, Miron VV. Aluminum-induced alterations in Purinergic system parameters of BV-2 brain microglial cells. J Immunol Res. 2020. 2021: 2695490
4. Back SH, Noh AR, Kim KA, Akram M, Shin YJ, Kim KA. Modulation of mitochondrial function and autophagy mediates carnosine neuroprotection against ischemic brain damage. Stroke. 2014. 45: 2438-43
5. Batanauskaite J, Sadauskiene I, Liekis A, Kasauskas A, Lazauskas R, Zlabiene U. Natural compounds rosmarinic acid and carvacrol counteract aluminum-induced oxidative stress. Molecules. 2020. 25: 1807
6. Bae ON, Serfozo K, Baek SH, Lee KY, Dorrance A, Rumbeiha W. Safety and efficacy evaluation of carnosine, an endogenous neuroprotective agent for ischemic stroke. Stroke. 2013. 44: 205-12
7. Bagheri H, Ghasemi F, Barreto GE, Rafiee R, Sathyapalan T, Sahebkar A. Effects of curcumin on mitochondria in neurodegenerative diseases. Biofactors. 2019. 46: 5-20
8. Beal MF. Does impairment of energy metabolism result in excitotoxic neuronal death in neurodegenerative illnesses?. Ann Neurol. 1992. 31: 119-30
9. Blaylock RL. A possible central mechanism in Autism Spectrum Disorders, Part 2: Immunoexcitotoxicity. Alter Ther Health Med. 2009. 15: 60-7
10. Blaylock RL. Why immunoexcitoxicity is the basis of most neurodegenerative diseases and systemic immune activation: An analysis. Surg Neurol Int. 2023. 14: 281
11. Blaylock RL. Aluminum induced immunoexcitoxicity and neurodevelopmental and neurodegenerative disorders. Curr Inorgic Chem. 2012. 2: 46-53
12. Blaylock RL, Maroon J. Immunoexcitotoxicity as a central mechanism in chronic traumatic encephalopathy-a unifying hypothesis. Surg Neurol Int. 2011. 2: 107
13. Becaria A, Lahiri DK, Bondy SC, Chen D, Hamadeh A, Li H. Aluminum and copper in drinking water enhance inflammatory or oxidatve events specifically in the brain. 2006. 176: 16-23
14. Block ML, Hong JS. Chronic microglial activation and progressive dopaminergic neurotoxicity. Biochem Soc Trans. 2007. 35: 1127-32
15. Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: Uncovering the molecular mechanisms. Nat Rev Neurosci. 2007. 8: 57-69
16. Bondy SC. The neurotoxicity of environmental aluminum is still an issue. Neurotoxicology. 2010. 35: 575-81
17. Boyman L, Karbowski M, Lederer WJ. Regulation of mitochondrial ATP production: Ca2+ signaling and quality control. Trends Mol Med. 2020. 26: 21-39
18. Brown GC, Neher JJ. Inflammatory neurodegeneration and mechanisms of microglial killing of neurons. Mol Neurobiol. 2010. 41: 242-7
19. Burton NC, Guilarte TR. Manganese neurotoxicity: Lessons learned from longitudinal studies in nonhuman primates. Environ Health Perspect. 2009. 117: 325-32
20. Bygrave FL. Mitochondria and control of intracellular calcium. Biol Rev. 1978. 53: 43-79
21. Campbell A, Becaria A, Lahiri DK, Sharman K, Bondy SC. Chronic exposure to aluminum in drinking water increases inflammatory parameters selectively in the brain. J Neurosci Res. 2004. 75: 565-72
22. Charleston JS, Body RL, Bolender RP, Mottet NK, Vahter ME, Burbacher TM. Changes in the number of astrocytes and microglia in the thalamus of the monkey Macaca fascicularis following long-term subclinical methylmercury exposure. Neurotoxicology. 1996. 17: 127-38
23. Chin-Chan M, Narvarro-Yepes J, Quintanilla-Vega B. Enviromental pollutants as risk factors for neurodegenerative disorders: Alzheimer’s and Parkinson’s diseases. Front Cell Neurosci. 2015. 9: 124
24. Cosgrove KE, Galvan EJ, Barrionuevo G, Meriney SD. mGluRs modulate strength and timing of excitatory transmission in hippocampal area CA3. Mol Neurobiol. 2011. 44: 93-101
25. Couette M, Boisse MF, Maison P, Brugieres P, Cesaro P, Chevalier X. Long-term persistence of vaccine-derived aluminum hydroxide is associated with chronic cognitive dysfunction. J. Inorg Biochem. 2009. 1: 1571-8
26. Cunat L, Lanhers MC, Joyeux M, Burnel D. Bioavailability and intestinal absorption of aluminum in rats: Effects of aluminum compounds and some dietary constituents. Biol Trace Elem Res. 2000. 76: 31-55
27. Cunningham C, Campion S, Lunnon K, Murray CL, Woods JF, Deacon RM. Systemic inflammation induces acute behavioral and cognitive changes and accelerates neurodegenerative disease. Biol Psychiatry. 2009. 6: 304-12
28. Cunningham C, Campion S, Teeling J, Felton L, Perry VH, editors. The sickness behavior and CNS inflammatory mediator profile induced by systemic challenge of mice with synthetic double- stranded RNA (Poly I: C). Brain Behav Immun. 2007. 21: 490-502
29. Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain Behav Immun. 2007. 21: 153-60
30. Deloncle R, Guillard O, Huguet F, Clanet F. Modification of the blood-brain barrier through chronic intoxication by aluminum glutamate. Possible role in the etiology of Alzheimer’s disease. Biol Trace Elem Res. 1995. 47: 227-33
31. El-Rahman SS. Neuropathology of aluminum toxicity in rats (glutamate and GABA impairment). Pharmacol Res. 2003. 47: 189-94
32. Esiri MM, Wilcock GK. The olfactory bulbs in Alzheimer’s disease. J Neurol Neurisurg Psychiatry. 1984. 47: 56-60
33. Evans PH, Yano E, Klinowski J, Peterhans E. Oxidative damage in Alzheimer’s dementia, and the potential etiopathogenic role of aluminosilicates, microglia and micronutrient interactions. EXS. 1992. 62: 178-89
34. Exley C. A molecular mechanism of aluminum-induced Alzheimer’s disease. J Inorg Biochem. 1999. 76: 133-40
35. Exley C. The pro-oxidant activity of aluminum. Free Rad Biol. 2004. 36: 380-7
36. Exley C, House E. Aluminum in the human brain. Monatsh Chem. 2011. 142: 357-63
37. Exley C, Siesjo P, Eriksson H, editors. The immunobiology of aluminum adjuvants: How do they really work?. Trends Immunol. 2010. 31: 103-9
38. Flarend RE, Hem SL, White JL, Elmore D, Suckow MA, Rudy AC. In vivo absorption of aluminium-containing vaccine adjuvants using 26Al. Vaccine. 1997. 15: 1314-8
39. Frey A, Neutra MR, Robey FA. Peptomer aluminum oxide nanoparticle conjugates as systemic and mucosal vaccine candidates: Synthesis and characterization of a conjugate derived from the C4 domain of HIV-1MN gp120. Bioconjug Chem. 1997. 8: 424-33
40. Garay PA, McAllister AK. Novel roles for immune molecules in neural development: Implications for neurodevelopmental disorders. Front Synaptic Neurosci. 2010. 2: 136
41. Gherardi RK, Cadusseau J, Authier FJ, Shoenfeld Y, editors. Aluminum particle biopersistence, systemic transport and long-term safety: Macrophagic myofasciitis and beyond. Vaccines and autoimmunity. United States: Wiley; 2015. p. 261-70
42. Greenamyre JT. Neuronal bioenergetic defects, excitotoxicity and Alzheimer’s disease: “Use it and lose it”. Neurobiol Aging. 1991. 12: 334-36 discussion 352-5
43. Griesmaier E, Keller M. Glutamate receptors-Prenatal insults, long-tern consequences. Pharm Biochem Behav. 2012. 100: 835-40
44. Gulya K, Rakonczay Z, Kasa P. Cholinotoxic effects of aluminum in rat brain. J Neurochem. 1990. 54: 1020-6
45. Hammound GM, Shalaby RA. Experimental evaluation of protective action of resveratrol against aluminum-induced toxicity in male rats. Int J Res Biol Sci. 2019. 6: 11-24
46. Harry GJ, Kraft AD. Neuroinflammation and microglia: Considerations and approaches for neurotoxicity assessment. Expert Opin Drug Metab Toxicol. 2008. 4: 1265-77
47. Izumi Y, Zorumski CF. Neuroprotective effects of pyruvate following NMDA-mediated excitotoxic insults in hippocampal slices. Neurosci Lett. 2010. 478: 131-5
48. Jahedsani A, Kherzi S, Ahangari M, Bakhshii S, Salimi A. Apigenin attenuates aluminum phosphide-induced cytotoxicity via reducing mitochondrial, lysosomal damages and oxidative stress in rat cardiomyosites. Pesitic Biochem Physiol. 2020. 167: 104585
49. Johnson VJ, Sharma RP, editors. Aluminum disrupts the pro- inflammatory cytokine/neurotrophin balance in primary brain rotation-mediated aggregate cultures: Possible role in neurodegeneration. Neurotoxicology. 2003. 24: 261-8
50. Justin-Thenmozhi A, Bharathi MD, Kiruthika R, Manivavasagam T, Borah A, Essa MM. Attenuation of aluminum-chloride-induced neuroinflammation and caspase activation through AKT/GSK-3ß pathway by hesperidin in Wistar rats. Neurotox Res. 2018. 34: 463-76
51. Komuro H, Rakic P. Modulation of neuronal migration by NMDA receptors. Science. 1993. 260: 95-7
52. Kreutzberg GW. Microglia: A sensor for pathological events in the CNS. Trends Neurosci. 1996. 19: 312-31
53. Kruck TP, Cui JG, Percy ME, Lukiw WJ. Molecular shuttle chelation: The use of ascorbate, desferrioxamine and Feralex-G in combination to remove nuclear bound aluminum. Cell Mol Neurobiol. 2004. 24: 443-59
54. Kumar V, Bal A, Gill KD, editors. Impairment of mitochondrial energy metabolism in different regions of rat brain following chronic exposure to aluminum. Brain Res. 2008. 1232: 94-103
55. Kwon JT, Seo GB, Jo E, Lee M, Kim HM, Shim I. Aluminum nanoparticles induces ERK and p38MAPK activation in rat brain. Toxicol Res. 2013. 29: 181-5
56. LaFerla FM. Calcium dyshomeostasis and intracellular signalling in Alzheimer’s disease. Nat Rev Neurosci. 2002. 3: 862-72
57. Lawson LJ, Perry VH, Dri P, Gordon S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. J Neurosci. 1990. 39: 151-70
58. Li X, Zheng H, Zhang Z, Li M, Huang Z, Schluesener HJ. Glia activation induced by peripheral administration of aluminum oxide nanoparticles in rat brains. Nanomedicine. 2009. 5: 473-9
59. Liu Z, Yu Y, Li X, Ross CA, Smith WW. Curcumin protects against Alpha-T alpha-synuclein-induced toxicity in a PC12 inducable cell model for Parkinsonism. Pharmacol Res. 2011. 63: 439-44
60. Ludolph AC, Riepe M, Ullrich K. Excitotoxicity, energy metabolism and neurodegeneration. J Inherit Metab Dis. 1993. 16: 716-23
61. Lukiw WJ, Bazan NG. Aluminum triggers NFkB signalling, inflammatory and apoptoic gene expression in human neural cells. J Neurochem. 2002. 81: 101
62. Lyons-Weiler J, Blaylock RL. Revisiting excess diagnoses of illness and conditions in children whose parents provided informed permission to vaccinate them. Int J Vaccine Theory Pract Res. 2022. 2: 603-18
63. Mahmound M, Elsoadaa SS. Effects of ascorbic acid, biopropalis and Royal Jelly against aluminum toxicity in rats. J Nat Sci Res. 2013. 3: 102-12
64. Mander P, Brown GC. Activation of microglial NADPH oxidase is synergistic with glial iNOS expression in inducing neuronal death: A dual-key mechanism of inflammatory neurodegeneration. J Neuroinflammation. 2005. 2: 20
65. Matsugami TR, Tanemura K, Mieda M, Nakatomi R, Yamada K, Kondo T. From the Cover: Indispensability of the glutamate transporters GLAST and GLT1 to brain development. Proc Natl Acad Sci U S A. 2006. 103: 12161-6
66. Matyja E. Aluminum enhances glutamate-mediated neurotoxicity in organotypic cultures of rat hippocampus. Folia Neuropathol. 2000. 38: 47-53
67. McKee AS, Munks MW, MacLeod MK, Fleenor CJ, Van Rooijen N, Kappler JW. Alum induces innate immune responses through macrophage and mast cell sensors, but these sensors are not required for alum to act as an adjuvant for specific immunity. J Immunol. 2009. 183: 4403-14
68. Mehta A, Prabhakar M, Kumar P, Deshmukh R, Sharma PL. Excitoxicity: Bridge to various triggers in neurodegenerative disorders. Eur J Pharmocol. 2013. 698: 6-18
69. Mohan N, Alleyne T, Adogwa A. The effects of ingested aluminum on brain cytochrome oxidase activity. West Indian Med J. 2009. 58: 422-7
70. Monnet-Tschudi F, Zurich MG, Boschat C, Corbaz A, Honegger P. Involvement of environmental mercury and lead in the etiology of neurodegenerative diseases. Rev Environ Health. 2006. 21: 105-17
71. Morris AJ, Malbon CC. Physiological regulation of G protein-linked signaling. Physiol Rev. 1999. 79: 1373-430
72. Mosca F, Tritto E, Muzzi A, Monaci E, Bagnoli F, Iavarone C. Molecular and cellular signatures of human vaccine adjuvants. Proc Natl Acad Sci U S A. 2008. 105: 10501-6
73. Murakami K, Yoshino M. Aluminum decreases the glutathione regeneration by the inhibition of NADPisocitrate dehydrogenase in mitochondria. J Cell Biochem. 2004. 93: 1267-71
74. Nam DT, Arseneault M, Murthy V, Ramassamy C. Potential role of acrolein in neurodegeneration and in Alzheimer’s disease. Curr Mol Pharmacol. 2010. 3: 66-78
75. Nayak P, Chatterjee AK, editors. Effects of aluminum exposure on brain glutamate and GABA systems: An experimental study in rats. Food Chem Toxicol. 2001. 39: 1285-9
76. Niu PY, Niu Q, Zhang QL, Wang LP, HE SC, Wu P. Aluminum impairs rat neural cell mitochondria in vitro. Inter J Immunopathol Pharmacol. 2005. 18: 683-9
77. Odagaki Y, Nishi N, Koyama T. Functional coupling between metabotropic glutamate receptors and G proteins in rat brain membranes. Eur J Pharmacol. 1996. 300: 151-4
78. Page G, Khidir FA, Pain S, Barrier L, Fauconneau B, Guillard O. Group 1 metabotropic glutamate receptors activate the p70S6 kinase via both mammalian target of rapamycin (mTOR) and extracellular signal-regulated kinase (ERK1/2) signaling pathways in rat striatal and hippocampal synaptoneurosomes. Neurochem Int. 2006. 49: 413-21
79. Pardo CA, Vargas DL, Zimmerman AW. Immunity, neuroglia and neuroinflammation in autism. Int Rev Psychiatry. 2005. 17: 485-95
80. Perl DP, Moalem S. Aluminum and Alzheimer’s disease, a personal perspective after 25 years. J Alzheimers Dis. 2006. 9: 291-300
81. Perry VH. The influence of systemic inflammation on inflammation in the brain: Implications from chronic neurodegenerative diseases. Brain Behav Immun. 2004. 18: 407-13
82. Perry VH, Nicoll JA, Holmes C. Microglia in neurodegenerative disease. Nat Rev Neurol. 2010. 6: 193-201
83. Platt B, Fiddler G, Riedel G, Henderson Z. Aluminum toxicity in the rat brain: Histochemical and immunocytochemical evidence. Brain Res Bull. 2001. 55: 257-67
84. Poderoso JJ. The formation of peroxynitrite in the applied physiology of mitochondrial nitric oxide. Arch Biochem Biophys. 2009. 484: 214-20
85. Poyton RO, Ball KA, Castello PR. Mitochondrial generation of free radicals and hypoxic signaling. Trends Endocrin Metab. 2009. 20: 332-40
86. Prakash A, Shur B, Kumar A. Naringin protects memory impairment and mitochondrial oxidative damage against aluminum-induced neurotoxicity in rats. Inter J Neurosci. 2013. 123: 636-45
87. Reddy PH, Manczak M, Yin X, Grady MC, Mitchell A, Kandimalla R. Protective effects of a natural product, curcumin, against amyloid beta induced mitochondrial and synaptic toxicities in Alzheimer’s disease. J Invest Med. 2016. 64: 1220-34
88. Redhead K, Quinlan GJ, Das RG, Gutteridge JM, editors. Aluminum-adjuvanted vaccines transiently increase aluminum levels in murine brain tissue. Pharmacol Toxicol. 1992. 70: 278-80
89. Reusche E, Pilz P, Oberascher G, Linder B, Egensperger R, Gloeckner K. Subacute fatal aluminum encephalopathy after reconstructive otoneurosurgery: A case report. Hum Pathol. 2001. 32: 1136-40
90. Rothwell N, Allan S, Toulmond S. The role of interleukin 1 in acute neurodegeneration and stroke: Pathophysiological and therapeutic implications. J. Clin Invest. 1997. 10: 02648-52
91. Schlett K. Glutamate as a modulator of embryonic and adult neurogenesis. Curr Top Med Chem. 2006. 6: 949-60
92. Shaw CA. Aluminum as a CNS and immune system toxin across the life span. Adv Exp Med Biol. 2018. 1091: 53-83
93. Shaw CA, Petrik MS. Aluminum hydroxide injections lead to motor deficits and motor neuron degeneration. J Inorg Biochem. 2009. 103: 1555-62
94. Shaw CA, Li D, Tomljenovic L. Are there negative CNS impacts of aluminum adjuvants used in vaccines and immunotherapy. Immunotherapy. 2014. 6: 1055-71
95. Shaw CA, Tomljenovic L. Aluminum in the central nervous system (CNS): Toxicity in humans and animals, vaccine adjuvants and autoimmunity. Immunol Res. 2013. 56: 304-16
96. Sharma DR, Sunkaria A, Wani WY, Sharma RK, Verma D, Priyanka K. Quercetin protects against aluminum induced oxidative stress and promotes mitochondrial biogenesis via activation of PGC-1α signaling pathway. Neurotoxicology. 2015. 51: 116-37
97. Silva VS, Goncalves PP. The inhibitory effect of aluminum on the (Na+/K+) ATPase activity of rat brain cortex synaptosomes. J Inorg Biochem. 2003. 97: 143-50
98. Shalini B, Sharma JD. Beneficial effects of Emblia Officinalis on fluoride-induced toxicity on brain biochemical indexes and learning-memory in rats. Toxicol Int. 2015. 22: 35-9
99. Shirabe T, Irie K, Uchida M. Autopsy case of aluminum encephalopathy. Neuropathology. 2008. 22: 206-10
100. Stellwagen D, Beattie EC, Seo JY, Malenka RC. Differential regulation of AMPA receptor and GABA Receptor Trafficking by tumor necrosis factor-alpha. J Neurosci. 2005. 25: 3219-28
101. Storey E, Hyman BT, Jenkins B, Brouillet E, Miller JM, Rosen BR. 1-Methyl-4-phenylpyridinium produces excitotoxic lesions in rat striatum as a result of impairment of oxidative metabolism. J Neurochem. 1992. 58: 1975-8
102. Strunecka A, Strunecky O, Patocka J. Fluoride plus aluminum: Useful tools in laboratory investigations, but messengers of false information. Physiol Res. 2002. 51: 557-64
103. Strunecka A, Blaylock RL, Patocka J, Strunecky O. Immunoexcitoxicity as the central mechanism of etiopathology and treatment of autism spectrum disorders: A possible role of fluoride and aluminum. Surg Neurol Int. 2018. 9: 74
104. Suarez-Fernandez MB, Soldado AB, Sanz-Medel A, Vega JA, Novelli A, Fernandez-Sanchez MT. Aluminum-induced degeneration of astrocytes occurs via apoptosis and results in neuronal death. Brain Res. 1999. 835: 125-36
105. Sunderman FW. Nasal toxicity, carcinogenicity, and olfactory uptake of metals. Ann Clin Lab Sci. 2001. 31: 3-24
106. Takeuchi H. Neurotoxicity by microglia: Mechanisms and potential therapeutic strategy. Clin Exp Neuroimmunol. 2010. 1: 12-21
107. Takeuchi H, Jin S, Wang J, Zhang G, Kawanokuchi J, Kuno R. Tumor necrosis factor-alpha induces neurotoxicity via glutamate release from hemichannels of activated microglia in an autocrine manner. J Biol Chem. 2006. 281: 21362-8
108. Theiss C, Meller K. Aluminum impairs gap junctional intercellular communication between astroglial cells in vitro. Cell Tissue Res. 2002. 310: 143-54
109. Thomann PA, Santos VD, Tora P, Schonknecht P, Essig M, Schroder J. Reduced olfactory bulb and tract volume in early Alzheimer’s disease-A MRI study. Neurobiol Aging. 2009. 30: 838-41
110. Tomljenovic L, Shaw CA. Mechanism of aluminum toxicity and autoimmunity in pediatric populations. Lupus. 2012. 21: 223-30
111. Tomljenovic L. Aluminum and Alzheimer’s disease: After a century of controversy, is there a plausible link?. J Alzheimers Dis. 2011. 23: 567-98
112. Tomljenovic L, Shaw CA, editors. Aluminum vaccine adjuvants: Are they safe?. Curr Med Chem. 2011. 18: 2630-7
113. Town T, Nikolic V, Tan J. The microglial “activation” continuum: From innate to adaptive responses. J Neuroinflammation. 2005. 2: 24
114. Tsunoda M, Sharma RP. Modulation of tumor necrosis factor alpha expression in mouse brain after exposure to aluminum in drinking water. Arch Toxicol. 1999. 73: 419-26
115. Tyagi E, Agrawal R, Nath C, Shukla R. Inhibitory role of cholinergic system mediated via alpha7 nicotinic acetylcholine receptor in LPS-induced neuro-inflammation. Innate Immun. 2009. 16: 473-9
116. van Noort JM, Bsibsi M, editors. Toll-like receptors in the CNS: Implications for neurodegeneration and repair. Prog Brain Res. 2009. 175: 139-48
117. Walton JR. Aluminum in hippocampal neurons from humans with Alzheimer’s disease. Neurotoxicology. 2006. 27: 385-94
118. Walton J.R. An aluminum-based rat model for Alzheimer’s disease exhibits oxidative damage, inhibition of PP2A activity, hyperphosphorylated tau, and granulovacuolar degeneration. J Inorg Biochem. 2007. 101: 1275-84
119. Walton JR, Wang MX. APP expression, distribution and accumulation are altered by aluminum in a rodent model for Alzheimer’s disease. J Inorg Biochem. 2009. 103: 1548-54
120. Wigerblad G, Huie JR, Yin HZ, Leinders M, Pritchard RA, Koehrn FJ. Inflammation-induced GluA1 trafficking and membrane insertion of Ca2+ permeable AMPA receptors in dorsal horn neurons is dependent on spinal tumor necrosis factot, PI3 kinase and protein kinase A. Exp Neurol. 2017. 293: 144-58
121. Wood P. Microglia as a unique cellular target in the treatment of stroke: Potential neurotoxic medicators produce activated microglia. Neurol Res. 1995. 17: 242-8
122. Wu X, Li J, Hu JN, Deng ZY. Effects of glutamate and citrate on absorption and distribution of aluminum in rats. Biol Trace Elem Res. 2012. 148: 83-90
123. Yasuda Y, Shinagawa R, Yamada M, Mori T, Tateishi N, Fujita S. Long-lasting reactive changes observed in microglia in the striatal and substantia nigral of mice after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Brain Res. 2007. 1138: 196-202
124. Yawata I, Takeuchi H, Doi Y, Liang J, Mizuno T, Suzumura A. Macrophage-induced neurotoxicity is mediated by glutamate and attenuated by glutaminase inhibitors and gap junction inhibitors. Life Sci. 2008. 82: 1111-6
125. Yin Z, Milatovic D, Aschner JL, Syversen T, Rocha JB, Souza DO. Methylmercury induces oxidative injury, alterations in permeability and glutamine transport in cultured astrocytes. Brain Res. 2007. 1131: 1-10
126. Yu L, Zhi Q, Wang G, Zhang Q, Zhao J, Narbad A. Lactobacillus plantarum CCFM639 alleviates aluminum toxicity. App Microbiol Biotech. 2016. 100: 1891-900
127. Zhu X, Hao W, Liu Z, Song Y, Hao C, Wu S. Aluminum induces neuroinflammation via P2X7 receptor activating NLRP3 inflammasome pathway. Ecotoxicol Environ Saf. 2013. 249: 114373