- Department of Plastic Surgery, Hospital da Restauração, Recife, Pernambuco, Brazil
- Department of Neurosurgery, Hospital da Restauração, Recife, Pernambuco, Brazil
- Department of Plastic Surgery, Baylor College of Medicine, Houston, Texas, USA
Correspondence Address:
Juan Pablo Borges Rodrigues Maricevich
Department of Neurosurgery, Hospital da Restauração, Recife, Pernambuco, Brazil
DOI:10.4103/sni.sni_102_18
Copyright: © 2018 Surgical Neurology International This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.How to cite this article: Juan Pablo Borges Rodrigues Maricevich, Auricelio Batista Cezar, Edilson Xavier de Oliveira, Jose Arthur Morais Veras e Silva, Renata Souza Maricevich, Nivaldo Sena Almeida, Hildo Rocha Cirne Azevedo-Filho. Adhesion sutures for seroma reduction in cranial reconstructions with polymethyl methacrylate prosthesis in patients undergoing decompressive craniectomy: A clinical trial. 22-Aug-2018;9:168
How to cite this URL: Juan Pablo Borges Rodrigues Maricevich, Auricelio Batista Cezar, Edilson Xavier de Oliveira, Jose Arthur Morais Veras e Silva, Renata Souza Maricevich, Nivaldo Sena Almeida, Hildo Rocha Cirne Azevedo-Filho. Adhesion sutures for seroma reduction in cranial reconstructions with polymethyl methacrylate prosthesis in patients undergoing decompressive craniectomy: A clinical trial. 22-Aug-2018;9:168. Available from: http://surgicalneurologyint.com/surgicalint-articles/8985/
Abstract
Background:Cranial reconstruction with polymethyl methacrylate (PMMA) prosthesis is used for calvarial defects secondary to decompressive craniectomies. Seroma is one of the most frequent complications of this procedure and can lead to the dehiscence, extrusion, infection, and loss of the prosthesis. The objective of the study is to analyze the effectiveness of the tacking sutures between the prosthesis and the scalp flap in reducing the seroma.
Methods:This is a prospective study with 63 patients submitted to cranioplasty between 2014 and 2017 for defects resulting from decompressive craniectomies. All patients were followed up postoperatively for at least 3 months and the diagnosis of seroma was made clinically. In the first 22 patients, the conventional technique was applied and, in the following 41, the technique with tacking sutures was used. The incidence of seroma was collected for both groups.
Results:The overall incidence of seroma was 65.1%. Compared to the conventional technique, the use of tacking sutures was associated with a statistically significant reduction in the incidence of seroma from 90.9% to 51.2% (P = 0.002).
Conclusion:The use of the tacking sutures in cranioplasties with PMMA prosthesis reduced the incidence of seroma postoperatively.
Keywords: Cranial reconstruction, cranioplasty, descompressive craniectomy, polymethyl methacrylate, seroma
INTRODUCTION
Decompressive craniectomy is indicated for the treatment of severe and refractory intracranial hypertension related to conditions such as head trauma and ischemic stroke.[
Cranioplasty is associated with possible complications occurring in 19.7%–36.5% of cases.[
Our initial analysis of patients undergoing cranioplasty in our institution showed a high incidence of seroma, for which a change in technique was adopted. The tacking suture of Baroudi[
This study aims to demonstrate the effectiveness of the tacking sutures applied to cranial reconstructions with polymethyl methacrylate (PMMA) prosthesis in reducing the incidence of seroma.
METHODS
This study was approved by the Hospital of Restauração (HR) IRB-CAAE under the number 128551/2017. This was a retrospective review of all patients who underwent cranioplasty with PMMA after decompressive craniectomy as a consequence of severe head trauma, stroke, or neoplasia at the Plastic Surgery and Neurosurgery Services of the Hospital of Restauração (HR) in Recife, Brazil between 2014 and 2017. The study included 63 patients who were followed up for at least 3 months postoperatively.
Patients were evaluated for functional improvement, aesthetics, and complications. After initial data analysis of 22 patients, a very high incidence of seroma was noted. Therefore, the surgical team adopted a new intervention.
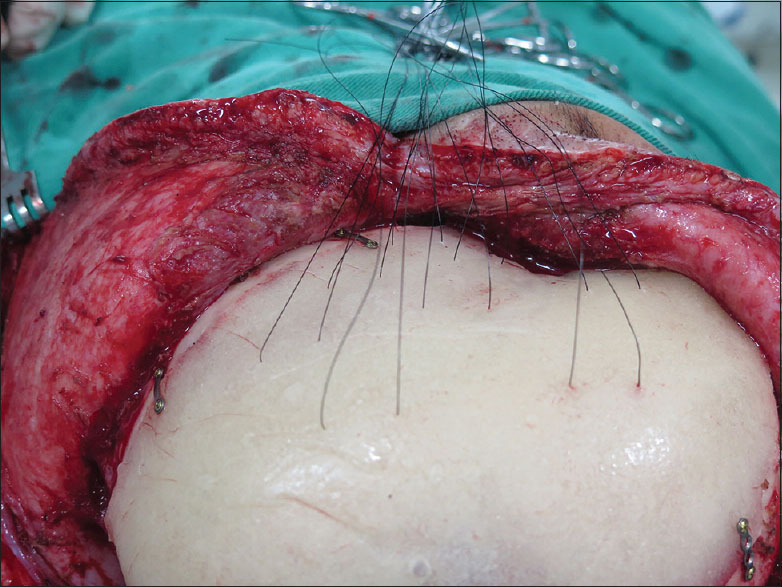
The customized PMMA prosthesis was prepared intraoperatively with the aid of three-dimensional (3D) models, and then placed in solution with antibiotic and saline until it reached room temperature. Exposure was obtained through a secondary incision from previous craniectomy, without resection of the scar, to avoid tension during the closure. The defect was exposed with elevation of the scalp flap just superficial to the dura. The prosthesis was then fitted into the defect and secured using titanium plates and screws (Bioplate®, 1.6-hole 2-plate system).
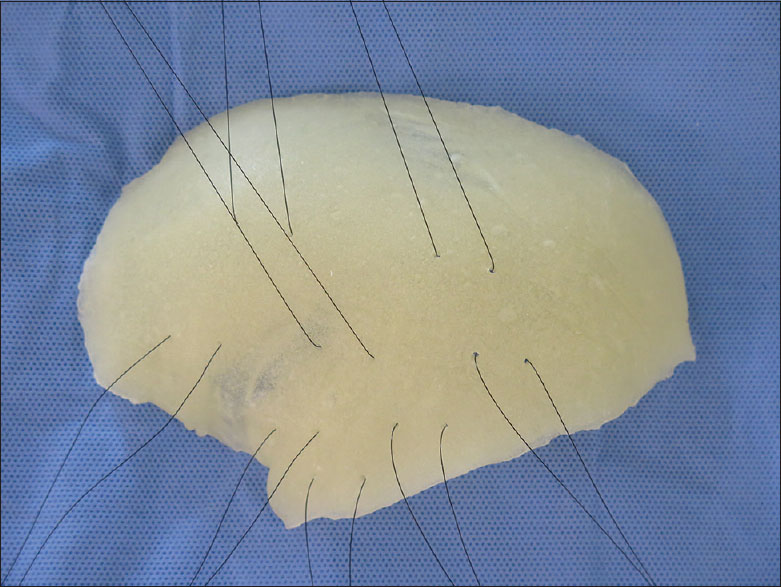
In the first 22 patients, the prosthesis was simply fixated and the closure was made in scalp layers. In the remaining 41 patients, before prosthesis fixation, fenestration was done with 8 pairs of holes and 3-0 Mononylon® sutures were threaded through them. All prostheses were routinely given 8 retention sutures or tacking sutures mainly in the temporal region [
A Blake® silicone tubular continuous suction drain was positioned and the surgical wound was closed in scalp layers. Postoperatively, the patient was referred to the Advanced Neurosurgery Support Unit (USAN) where a computed tomography (CT) scan was performed within the first 12 hours. The drain was removed when the output was lower than 50 ml per 12 hours, and hospital discharge usually occured within 48 hours.
The sample size was determined by the number of patients operated during the study period. The volume of the seroma was not calculated using imaging methods. The diagnosis was made by physical examination and all collection was punctured and emptied. Postoperative consultations were mandatorily carried out at 7, 14, 21, and 30 days and at the 3rd, 6th, and 12th month. The lowest emptied volume of one patient was 4 ml and the largest was 70 ml. Every time a patient was diagnosed with seroma, the patient was evaluated twice a week until resolution of the seroma with emptying and compressive dressing. All seromas were addressed with aspiration and drainage. Seroma was drained using a 40 × 12 needle and a 10 ml syringe only by the scar (less likely site of vascular injury). Chi-square test was used to calculate the proportions. P value of <0.05 was considered statistically significant.
RESULTS
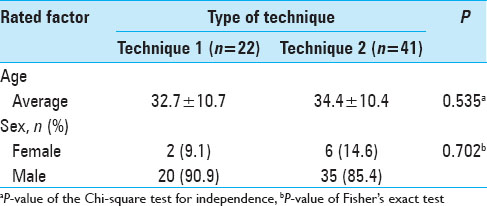
Sixty-three consecutive cranioplasties were performed in patients with an average age of 33 years (range, 13–58 years), including 55 males and 8 females. The average time between decompressive craniectomy and cranial reconstruction was 21 months, ranging from 2 to 84 months. The average area of the defect was 147 cm2. The average postoperative follow-up of the patients was 21 months (range, 6–33 months). There was significant statistical difference in age and gender between both groups (P value = 0.535 and 0.702, respectively) [
The overall incidence of seroma was 65.1%. However, there was a statistically significant decrease in the incidence from 90.9% to 51.2% (P = 0.002) after adopting the use of tacking sutures between the prosthesis and scalp flap. All patients who developed seroma underwent aspiration and/or drainage. Contained seroma is a predisposing factor of infection. There was one case of extradural hematoma after seroma aspiration on postoperative day 10 requiring immediate evacuation.
Seromas usually develop between the 1st and 2nd week of surgeries, whereas extradural hematomas usually develop within the first 48 hours while the patient is still hospitalized. In the aspirated contents, it is also possible to differentiate the characteristics of the liquid between seroma and hamatoma. The resolution of the seroma in the postoperative period was assessed clinically (inspection and palpation) [
DISCUSSION
As part of a larger study on the use of customized PMMA prosthesis for calvarial reconstruction, we initially found a prohibitive incidence of seroma. Therefore, we intervened with the application of the Baroudi's tacking sutures[
The large tissue detachment, residual dead space, and the presence of alloplastic material are contributory factors for seroma formation. Seroma, if not drained, may evolve to wound dehiscence, implant extrusion, infection, and, finally, loss of reconstruction. On the other hand, serial seroma aspirations carry the risk of introducing infection and vascular injury.
Our seroma rate was higher than that reported in the literature. Williams et al. (N = 151) reported an incidence of 14.5% with titanium prosthesis[
The use of tacking sutures between the scalp flap and the prosthesis is a reproducible technique that does not add considerably to the surgical time. In plastic surgery operations, it is an already well-defined strategy for the reduction of seroma.[
CONCLUSION
The creation of tacking sutures between the scalp flap and the prosthesis, a simple and reproducible technique, statistically reduced the incidence of seromas in cranial reconstructions with PMMA prosthesis through 3D impression molds.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Aarabi B, Hesdorffer DC, Ahn ES, Aresco C, Scalea TM, Eisenberg HM. Outcome following decompressive craniectomy for malignant swelling due to severe head injury. J Neurosurg. 2006. 104: 469-79
2. Agner C, Dujovny M, Gaviria M. Neurocognitive assessment before and after cranioplasty. Acta Neurochir (Wien). 2002. 144: 1033-40
3. Baroudi R, Ferreira CA. Seroma: How to avoid it and how to treat it. Aesthet Surg J. 1998. 18: 439-41
4. Broughton E, Pobereskin L, Whitfield PC. Seven years of cranioplasty in a regional neurosurgical centre. Br J Neurosurg. 2014. 28: 34-9
5. Bullock MR, Chesnut R, Ghajar J, Gordon D, Hartl R, Newell DW. Surgical management of acute subdural hematomas. Neurosurgery. 2006. 58: S16-24
6. Chang V, Hartzfeld P, Langlois M, Mahmood A, Seyfried D. Outcomes of cranial repair after craniectomy. J Neurosurg. 2010. 112: 1120-4
7. Chaouat M, Levan P, Lalanne B, Buisson T, Nicolau P, Mimoun M. Abdominal dermolipectomies: Early postoperative complications and long-term unfavorable results. Plast Reconstr Surg. 2000. 106: 1614-8
8. Daltrey I, Thomson H, Hussien M, Krishna K, Rayter Z, Winters ZE. Randomized clinical trial of the effect of quilting latissimus dorsi flap donor site on seroma formation. Br J Surg. 2006. 93: 825-30
9. De Bonis P, Frassanito P, Mangiola A, Nucci CG, Anile C, Pompucci A. Cranial repair: How complicated is filling a “hole”?. J Neurotrauma. 2012. 29: 1071-6
10. Di Stefano C, Sturiale C, Trentini P, Bonora R, Rossi D, Cervigni G. Unexpected neuropsychological improvement after cranioplasty: A case series study. Br J Neurosurg. 2012. 26: 827-31
11. Gooch MR, Gin GE, Kenning TJ, German JW. Complications of cranioplasty following decompressive craniectomy: Analysis of 62 cases. Neurosurg Focus. 2009. 26: E9-
12. Guerra WK, Gaab MR, Dietz H, Mueller JU, Piek J, Fritsch MJ. Surgical decompression for traumatic brain swelling: Indications and results. J Neurosurg. 1999. 90: 187-96
13. Güresir E, Beck J, Vatter H, Setzer M, Gerlach R, Seifert V. Subarachnoid hemorrhage and intracerebral hematoma: Incidence, prognostic factors, and outcome. Neurosurgery. 2008. 63: 1088-93
14. Güresir E, Raabe A, Setzer M, Vatter H, Gerlach R, Seifert V. Decompressive hemicraniectomy in subarachnoid haemorrhage: The influence of infarction, haemorrhage and brain swelling. J Neurol Neurosurg Psychiatry. 2009. 80: 799-801
15. Honeybul S, Ho KM, Lind CR, Corcoran T, Gillett GR. The retrospective application of a prediction model to patients who have had a decompressive craniectomy for trauma. J Neurotrauma. 2009. 26: 2179-83
16. Honeybul S, Janzen C, Kruger K, Ho KM. The impact of cranioplasty on neurological function. Br J Neurosurg. 2013. 27: 636-41
17. Janzen C, Kruger K, Honeybul S. Syndrome of the trephined following bifrontal decompressive craniectomy: Implications for rehabilitation. Brain Inj. 2012. 26: 101-5
18. Schwab S, Steiner T, Aschoff A, Schwarz S, Steiner HH, Jansen O. Early hemicraniectomy in patients with complete middle cerebral artery infarction. Stroke. 1998. 29: 1888-93
19. Sobani ZA, Shamim MS, Zafar SN, Qadeer M, Bilal N, Murtaza SG. Cranioplasty after decompressive craniectomy: An institutional audit and analysis of factors related to complications. Surg Neurol Int. 2011. 2: 123-
20. Srivastava V, Basu S, Shukla VK. Seroma formation after breast cancer surgery: What we have learned in the last two decades. J Breast Cancer. 2012. 15: 373-80
21. Stanczyk M, Grala B, Zwierowicz T, Maruszynski M. Surgical resection for persistent seroma, following modified radical mastectomy. World J Surg Oncol. 2007. 5: 104-
22. Williams LR, Fan KF, Bentley RP. Custom-made titanium cranioplasty: Early and late complications of 151 cranioplasties and review of the literature. Int J Oral Maxillofac Surg. 2015. 44: 599-608