- Department of Neurosurgery, Division of Pediatric Neurosurgery, Baylor College of Medicine/Texas Children’s Hospital, Houston, Texas,
- Department of Neurosurgery, Division of Pediatric Neurosurgery, Northwestern University Feinberg School of Medicine/Ann and Robert H Lurie Children’s Hospital, Chicago, IL, USA.
Correspondence Address:
Sandi K. Lam
Department of Neurosurgery, Division of Pediatric Neurosurgery, Northwestern University Feinberg School of Medicine/Ann and Robert H Lurie Children’s Hospital, Chicago, IL, USA.
DOI:10.25259/SNI_418_2019
Copyright: © 2019 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Nisha Gadgil, Matthew Muir, Melissa A. Lopresti, Sandi K. Lam. An update on pediatric surgical epilepsy: Part II. 27-Dec-2019;10:258
How to cite this URL: Nisha Gadgil, Matthew Muir, Melissa A. Lopresti, Sandi K. Lam. An update on pediatric surgical epilepsy: Part II. 27-Dec-2019;10:258. Available from: https://surgicalneurologyint.com/surgicalint-articles/9820/
Abstract
Background: Recent advances may allow surgical options for pediatric patients with refractory epilepsy not previously deemed surgical candidates. This review outlines major technological developments in the field of pediatric surgical epilepsy.
Methods: The literature was comprehensively reviewed and summarized pertaining to stereotactic electroencephalography (sEEG), laser ablation, focused ultrasound (FUS), responsive neurostimulation (RNS), and deep brain stimulation (DBS) in pediatric epilepsy patients.
Results: sEEG allows improved seizure localization in patients with widespread, bilateral, or deep-seated epileptic foci. Laser ablation may be used for destruction of deep-seated epileptic foci close to eloquent structures; FUS has a similar potential application. RNS is a palliative option for patients with eloquent, multiple, or broad epileptogenic foci. DBS is another palliative approach in children unsuitable for respective surgery.
Conclusion: The landscape of pediatric epilepsy is changing due to improved diagnostic and treatment options for patients with refractory seizures. These interventions may improve seizure outcomes and decrease surgical morbidity, though further research is needed to define the appropriate role for each modality.
Keywords: Epilepsy surgery, Innovation, Minimally invasive, Pediatric, Technology
INTRODUCTION
Pediatric epilepsy has a worldwide prevalence of 1%, and 20–30% are diagnosed with drug-resistant epilepsy (DRE) (persistent seizures despite treatment with two first-line antiepileptic medications).[
Recent developments allow surgical options for patients previously not deemed candidates, such as those with bilateral, deep, eloquent, or poorly localizing epileptogenic foci. The American Academy of Neurology now recommends early surgery for select patients with DRE. Nevertheless, there is still substantial delay between diagnosis and surgical referral.[
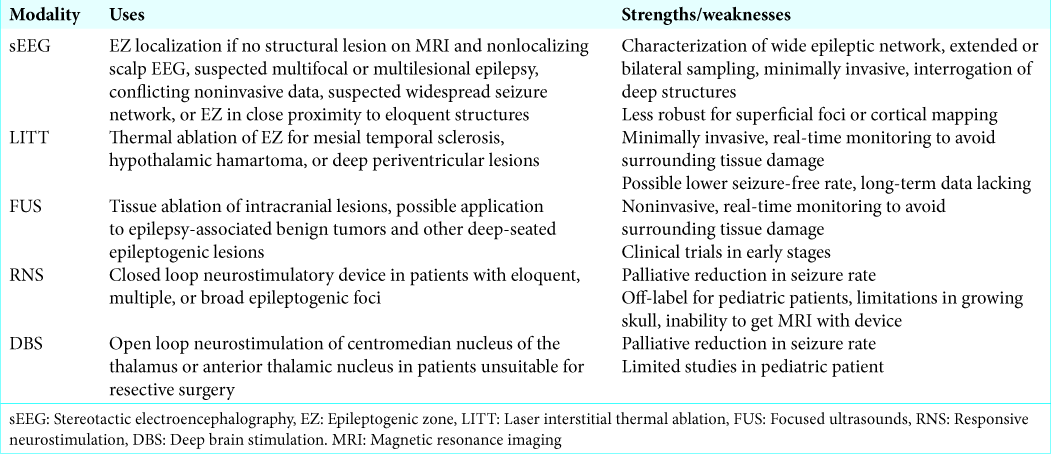
sEEG
Complete removal of the epileptogenic zone (EZ) is the most important factor associated with postsurgical seizure freedom. Accurate localization of the EZ is therefore critical, as is information obtained from semiology, EEG, structural magnetic resonance imaging (MRI), and other advanced testing.[
There are two main types of intracranial electrode monitoring: subdural strip/grid recordings, and stereotactically placed depth electrodes implanted through burr holes (sEEG). Robotic assistance has a 1–3 mm level of accuracy for placement of electrodes and allows safe trajectories with reduced operating time [
Patients with DRE may be considered for sEEG in the following situations: no structural lesion identified on MRI and scalp EEG nonlocalizing, suspected multifocal/multilesional epilepsy, conflicting noninvasive data, suspected widespread seizure network, or EZ in close proximity to eloquent structures.[
sEEG is a minimally invasive approach for seizure localization that provides significant advantages over subdural electrodes such as sampling of extended regions, interrogation of deep structures not accessible to subdural electrodes,[
LASER ABLATION
After the seizure focus has been appropriately localized, there are multiple surgical options, including open surgical resection and minimally invasive stereotactic techniques such as radiofrequency thermo-coagulation and stereotactic radiosurgery. However, radiofrequency thermo-coagulation does not allow real-time monitoring of tissue destruction and is less effective than open microsurgical resection.[
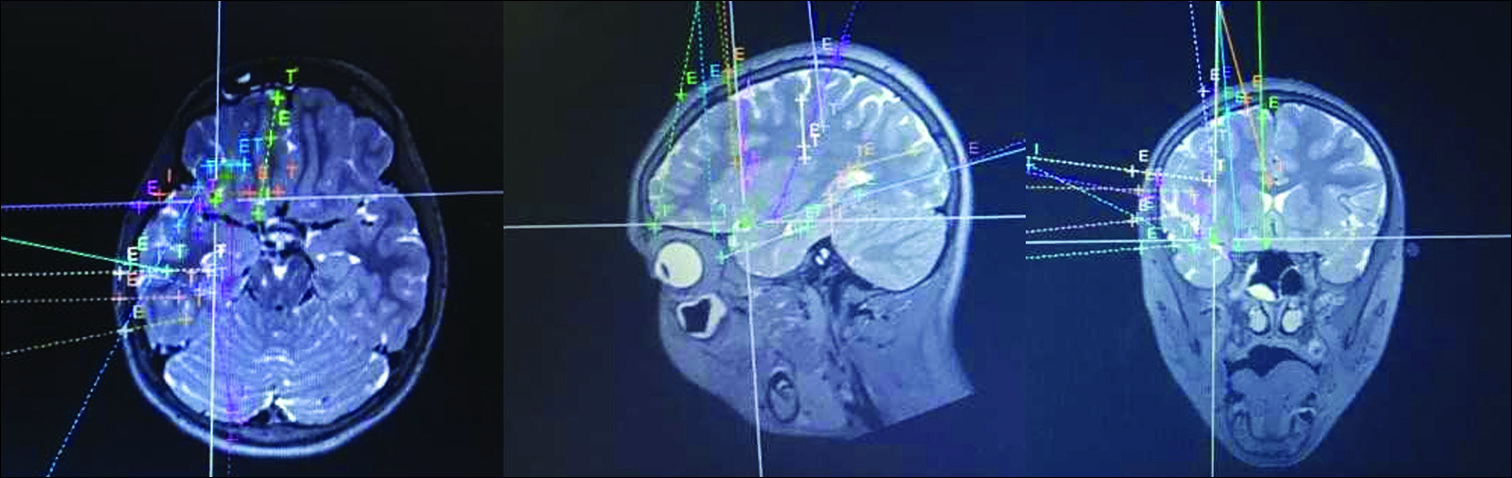
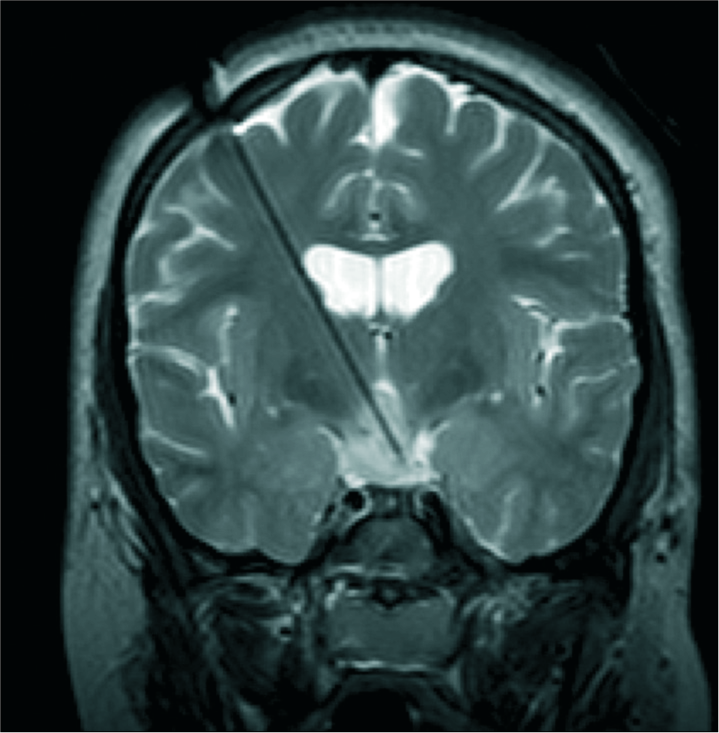
MR-guided LITT is used to treat mesial temporal sclerosis, hypothalamic hamartoma, and deep periventricular lesions.[
In pediatrics, hypothalamic hamartoma is the most frequently reported indication for LITT, allowing disconnection of these deep-seated lesions while avoiding damage to surrounding structures [
Figure 2:
Intraoperative T2-weighted coronal magnetic resonance imaging of a child undergoing laser ablation for intractable gelastic epilepsy resulting from hypothalamic hamartoma. The laser cannula is in place with the tip terminating in the hamartoma, allowing focused delivery of heat that can be monitored in real-time using magnetic resonance thermography.
FUS
FUS is another minimally invasive modality used to create targeted tissue ablation by delivering high-intensity ultrasound waves through external transducer elements which cause irreversible coagulation. FUS creates a 2–6 mm diameter intracranial lesion with 1 mm precision. Tissue ablation can be monitored in real-time using MR thermography. FUS avoids the need for skin incision or burr holes and carries a reduced complication rate compared to open microsurgery or LITT.[
The current primary application of FUS is for treating essential tremor, Parkinson’s disease, and neuropathic pain, and more recently, deep brain tumors.[
RNS
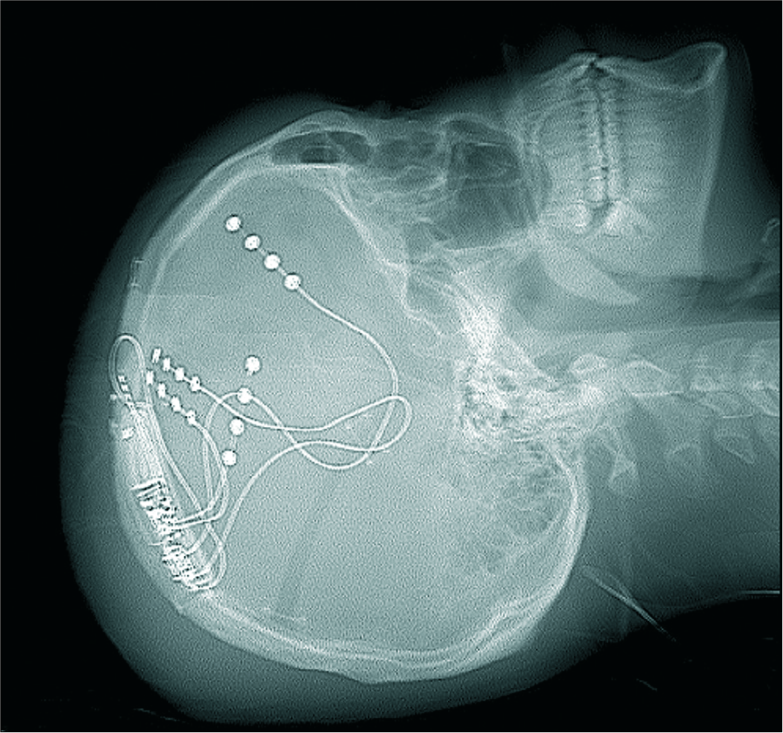
RNS adapts therapeutic stimulation in response to a continuous feedback loop. Depth or strip electrodes within the ictal onset zone continuously monitor electrocorticography activity; the device uses a programmed algorithm to detect incipient seizures. Once an abnormal activity is detected, the device supplies responsive therapeutic electrical stimulation designed to reduce or abort the seizures.[
NeuroPace RNS (Neuropace, Mountain View, CA) is the first and only FDA-approved RNS device available to patients aged 18 years or older.[
RNS has been used off-label in pediatric patients with DRE in those with no surgical resection options (bilateral or eloquent epileptogenic foci).[
An example of RNS placement is demonstrated in
DEEP BRAIN STIMULATION (DBS)
DBS is an open-loop neuromodulatory program that involves the delivery of electrical stimulation to deep brain structures through implanted electrodes connected to a pulse generator. The safety profile is similar to DBS for movement disorders.[
CONCLUSION
Advances in diagnostic capabilities and minimally invasive treatments, including stereotaxy, surgical robotics, laser ablation, and neurostimulation may improve seizure outcomes while minimizing surgical morbidity.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Abe K, Taira T. Focused ultrasound treatment, present and future. Neurol Med Chir (Tokyo). 2017. 57: 386-91
2. Berg AT, Jallon P, Preux PM. The epidemiology of seizure disorders in infancy and childhood: Definitions and classifications. Handb Clin Neurol. 2013. 111: 391-8
3. Buckley R, Estronza-Ojeda S, Ojemann JG. Laser ablation in pediatric epilepsy. Neurosurg Clin N Am. 2016. 27: 69-78
4. Bystritsky A, Korb AS, Douglas PK, Cohen MS, Melega WP, Mulgaonkar AP. A review of low-intensity focused ultrasound pulsation. Brain Stimul. 2011. 4: 125-36
5. Cossu M, Cardinale F, Castana L, Citterio A, Francione S, Tassi L. Stereoelectroencephalography in the presurgical evaluation of focal epilepsy: A retrospective analysis of 215 procedures. Neurosurgery. 2005. 57: 706-18
6. Cossu M, Cardinale F, Castana L, Nobili L, Sartori I, Lo Russo G. Stereo-EEG in children. Childs Nerv Syst. 2006. 22: 766-78
7. Curry DJ, Raskin J, Ali I, Wilfong AA. MR-guided laser ablation for the treatment of hypothalamic hamartomas. Epilepsy Res. 2018. 142: 131-4
8. Eekers DB, Pijnappel EN, Schijns OE, Colon A, Hoeben A, Zindler JD. Evidence on the efficacy of primary radiosurgery or stereotactic radiotherapy for drug-resistant non-neoplastic focal epilepsy in adults: A systematic review. Seizzure. 2018. 55: 83-92
9. Elias WJ, Lipsman N, Ondo WG, Ghanouni P, Kim YG, Lee W. A randomized trial of focused ultrasound thalamotomy for essential tremor. N Engl J Med. 2016. 375: 730-9
10. Englot DJ, Birk H, Chang EF. Seizure outcomes in nonresective epilepsy surgery: An update. Neurosurg Rev. 2017. 40: 181-94
11. Englot DJ, Breshears JD, Sun PP, Chang EF, Auguste KI. Seizure outcomes after resective surgery for extra-temporal lobe epilepsy in pediatric patients. J Neurosurg Pediatr. 2013. 12: 126-33
12. Englot DJ, Rolston JD, Wang DD, Sun PP, Chang EF, Auguste KI. Seizure outcomes after temporal lobectomy in pediatric patients. J Neurosurg Pediatr. 2013. 12: 134-41
13. Fisher R, Salanova V, Witt T, Worth R, Henry T, Gross R. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. 2010. 51: 899-908
14. Foley JL, Little JW, Vaezy S. Effects of high-intensity focused ultrasound on nerve conduction. Muscle Nerve. 2008. 37: 241-50
15. Guan J, Karsy M, Ducis K, Bollo RJ. Surgical strategies for pediatric epilepsy. Transl Pediatr. 2016. 5: 55-66
16. Heck CN, King-Stephens D, Massey AD, Nair DR, Jobst BC, Barkley GL. Two-year seizure reduction in adults with medically intractable partial onset epilepsy treated with responsive neurostimulation: Final results of the RNS system pivotal trial. Epilepsia. 2014. 55: 432-41
17. Hoppe C, Helmstaedter C. Laser interstitial thermotherapy (LiTT) in pediatric epilepsy surgery. Seizure. 2018. 18: S1059-311
18. Kang JY, Wu C, Tracy J, Lorenzo M, Evans J, Nei M. Laser interstitial thermal therapy for medically intractable mesial temporal lobe epilepsy. Epilepsia. 2016. 57: 325-34
19. Karsy M, Guan J, Ducis K, Bollo RJ. Emerging surgical therapies in the treatment of pediatric epilepsy. Transl Pediatr. 2016. 5: 67-78
20. Kinnear KM, Warner NM, Gersappe A, Doherty MJ. Pilot data on responsive epilepsy neurostimulation, measures of sleep apnea and continuous glucose measurements. Epilepsy Behav Case Rep. 2018. 9: 33-6
21. Knake S, Triantafyllou C, Wald LL, Wiggins G, Kirk GP, Larsson PG. 3T phased array MRI improves the presurgical evaluation in focal epilepsies: A prospective study. Neurology. 2005. 65: 1026-31
22. Kokoszka MA, Panov F, La Vega-Talbott M, McGoldrick PE, Wolf SM, Ghatan S. Treatment of medically refractory seizures with responsive neurostimulation: 2 pediatric cases. J Neurosurg Pediatr. 2018. 21: 421-7
23. Krishna V, Sammartino F, Rezai A. A review of the current therapies, challenges, and future directions of transcranial focused ultrasound technology: Advances in diagnosis and treatment. JAMA Neurol. 2018. 75: 246-54
24. Lang M, Chitale A, Sharan A, Wu C. Advancements in stereotactic epilepsy surgery: Stereo-EEG, laser interstitial thermotherapy, and responsive neurostimulation. JHN J. 2016. 11: 32-6
25. Li MC, Cook MJ. Deep brain stimulation for drug-resistant epilepsy. Epilepsia. 2018. 59: 273-90
26. Liava A, Mai R, Tassi L, Cossu M, Sartori I, Nobili L. Paediatric epilepsy surgery in the posterior cortex: A study of 62 cases. Epileptic Disord. 2014. 16: 141-64
27. MacDonell J, Patel N, Rubino S, Ghoshal G, Fischer G, Burdette EC. Magnetic resonance-guided interstitial high-intensity focused ultrasound for brain tumor ablation. Neurosurg Focus. 2018. 44: E11-
28. McGonigal A, Sahgal A, De Salles A, Hayashi M, Levivier M, Ma L. Radiosurgery for epilepsy: Systematic review and International Stereotactic Radiosurgery Society (ISRS) practice guideline. Epilepsy Res. 2017. 137: 123-31
29. Meador KJ, Kapur R, Loring DW, Kanner AM, Morrell MJ, RNS® System Pivotal Trial Investigators. Quality of life and mood in patients with medically intractable epilepsy treated with targeted responsive neurostimulation. Epilepsy Behav. 2015. 45: 242-7
30. Morrell MJ, RNS System in Epilepsy Study Group. Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology. 2011. 77: 1295-304
31. Pestana Knight EM, Schiltz NK, Bakaki PM, Koroukian SM, Lhatoo SD, Kaiboriboon K. Increasing utilization of pediatric epilepsy surgery in the United States between 1997 and 2009. Epilepsia. 2015. 56: 375-81
32. Ravindra VM, Sweney MT, Bollo RJ. Recent developments in the surgical management of paediatric epilepsy. Arch Dis Child. 2017. 102: 760-6
33. Rheims S, Fischer C, Ryvlin P, Isnard J, Guenot M, Tamura M. Long-term outcome of gamma-knife surgery in temporal lobe epilepsy. Epilepsy Res. 2008. 80: 23-9
34. Singhal NS, Numis AL, Lee MB, Chang EF, Sullivan JE, Auguste KI. Responsive neurostimulation for treatment of pediatric drug-resistant epilepsy. Epilepsy Behav Case Rep. 2018. 10: 21-4
35. Taussig D, Lebas A, Chipaux M, Jan M, Fohlen M, Bulteau C. Stereo-electroencephalography (SEEG) in children surgically cured of their epilepsy. Neurophysiol Clin. 2016. 46: 3-15
36. Thomas GP, Jobst BC. Critical review of the responsive neurostimulator system for epilepsy. Med Devices (Auckl). 2015. 8: 405-11
37. Tufail Y, Matyushov A, Baldwin N, Tauchmann ML, Georges J, Yoshihiro A. Transcranial pulsed ultrasound stimulates intact brain circuits. Neuron. 2010. 66: 681-94
38. Willie JT, Laxpati NG, Drane DL, Gowda A, Appin C, Hao C. Real-time magnetic resonance-guided stereotactic laser amygdalohippocampotomy for mesial temporal lobe epilepsy. Neurosurgery. 2014. 74: 569-84
39. Yan H, Toyota E, Anderson M, Abel TJ, Donner E, Kalia SK. A systematic review of deep brain stimulation for the treatment of drug-resistant epilepsy in childhood. J Neurosurg Pediatr. 2018. 23: 274-84