- Department of Neurosurgery, The Center For Advanced Neurology and Neurosurgery, Brazil (CEANNE),
- Department of Neurosurgery, Hospital São Lucas - PUCRS, Porto Alegre, Brazil.
Correspondence Address:
Eduardo Mello Rodrigues, Department of Neurosurgery, The Center For Advanced Neurology and Neurosurgery, Brazil (CEANNE), Porto Alegre, Brazil.
DOI:10.25259/SNI_1157_2021
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Eduardo Mello Rodrigues1, Gustavo Rassier Isolan1, Lia Grub Becker1, Leandro Infantini Dini1, Marco Antônio Schlindwein Vaz1, Thomas More Frigeri2. Anatomy of the optic radiations from the white matter fiber dissection perspective: A literature review applied to practical anatomical dissection. 22-Jul-2022;13:309
How to cite this URL: Eduardo Mello Rodrigues1, Gustavo Rassier Isolan1, Lia Grub Becker1, Leandro Infantini Dini1, Marco Antônio Schlindwein Vaz1, Thomas More Frigeri2. Anatomy of the optic radiations from the white matter fiber dissection perspective: A literature review applied to practical anatomical dissection. 22-Jul-2022;13:309. Available from: https://surgicalneurologyint.com/surgicalint-articles/11741/
Abstract
Background: Knowledge of the anatomical course of the optic radiations and its relationship to medial temporal lobe structures is of great relevance in preoperative planning for surgery involving the temporal lobe to prevent damage that may result in postsurgical visual field deficits.
Methods: In this anatomical study, we reviewed the literature on this topic and applied the information to practical anatomical dissection. The three-dimensional relationship between the course of the optic radiations and structures accessed in the main microneurosurgical approaches to the medial temporal lobe was examined by applying Klingler’s white matter fiber dissection technique to five formalin-fixed human brains. The dissections were performed with an operating microscope at magnifications of ×3–×40. High-resolution images were acquired during dissection for identification of the anatomical structures, focusing on the characterization of the course of the optic radiations in relation to medial temporal lobe structures.
Results: In all five dissected brains, we could expose and clearly define the relationship between the optic radiations and medial temporal lobe structures, improving our understanding of these complex structures.
Conclusion: The knowledge gained by studying these relationships will help neurosurgeons to develop risk-adjusted approaches to prevent damage to the optic radiations in the medial temporal region, which may result in a disabling visual field deficit.
Keywords: Fiber dissection, Neurosurgery, Optic radiations, Surgical approach, Temporal lobe, White matter
INTRODUCTION
The optic radiations are key white matter structures that cross the temporal lobe. Their extensive course involving the temporal, parietal, and occipital lobes makes them particularly susceptible to damage during neurosurgery, which may lead to visual field deficits.[
Fiber dissection was the first technique that allowed physicians to visualize the three-dimensional anatomic organization of the brain. In 1935, Klingler improved the technique, later named after him, by studying the specimens after formalin fixation, freezing, and defrosting, which facilitated the separation of fibers during dissection due to the formation of ice crystals between them.[
There are several surgical approaches to the temporal lobe currently in use, including the lateral, subtemporal, transsylvian, and supracerebellar transtentorial approaches.[
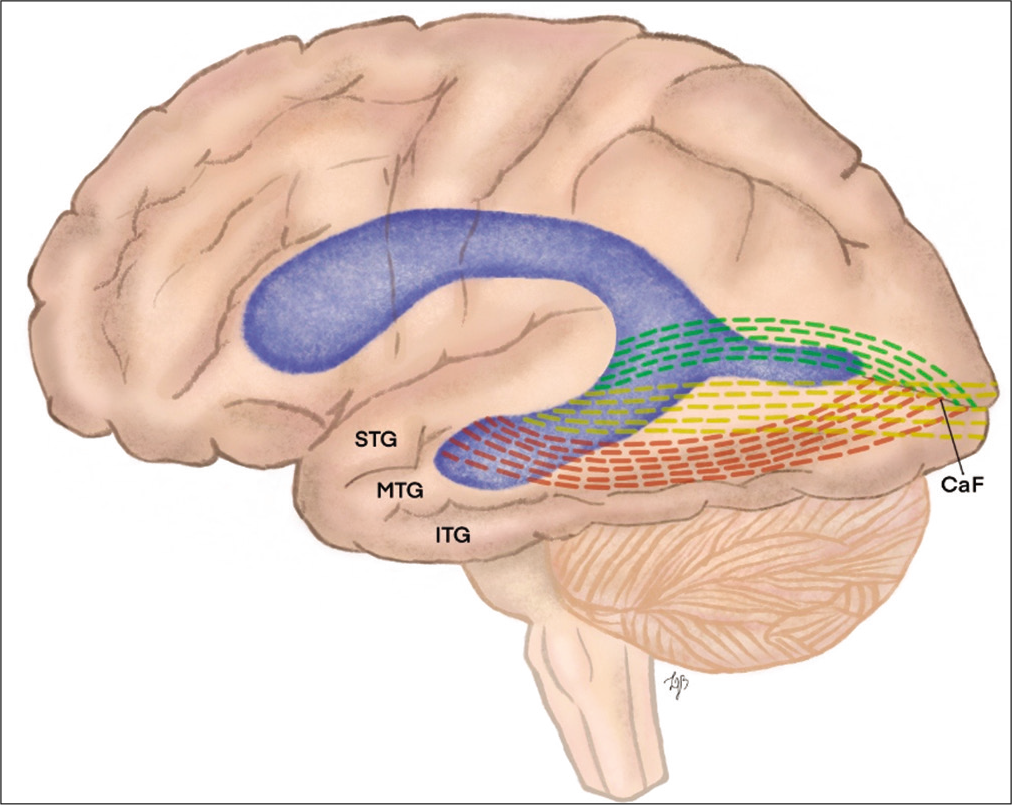
Figure 2:
Illustration demonstrating the relationship of optical radiation through the roof of the temporal horn to the calcarine fissure. STG: Superior temporal gyrus, MTG: middle temporal gyrus, ITG: inferior temporal gyrus, and Caf: calcarine fissure. Green dotted line: Meyer’s loop; yellow dotted line: central bundle ; red dotted line: dorsal bundle.
In this anatomical study, we reviewed the literature on this topic and applied the information to practical anatomical dissection by examining the three-dimensional relationship between the course of the optic radiations and structures accessed in the main microneurosurgical approaches to the medial temporal lobe (lateral, subtemporal, transsylvian, and supracerebellar transtentorial approaches) using Klingler’s white matter fiber dissection technique with the aid of an operating microscope.
MATERIALS AND METHODS
The study was approved by the Research Ethics Committee of Universidade Federal do Rio Grande do Sul, Brazil, and conducted in accordance with the provisions of the Declaration of Helsinki. Five human brains (ten hemispheres) were dissected. All specimens were prepared, dissected, and photographed in the Dr. Albert L. Rhoton NeuroMicroanatomy Laboratory at the University of Florida, USA.
Klingler’s white matter fiber dissection technique was applied to all brains.[
RESULTS
Under the operating microscope, the following observations were applied to all five dissected brains examined in the study.
Overview of the optic radiations
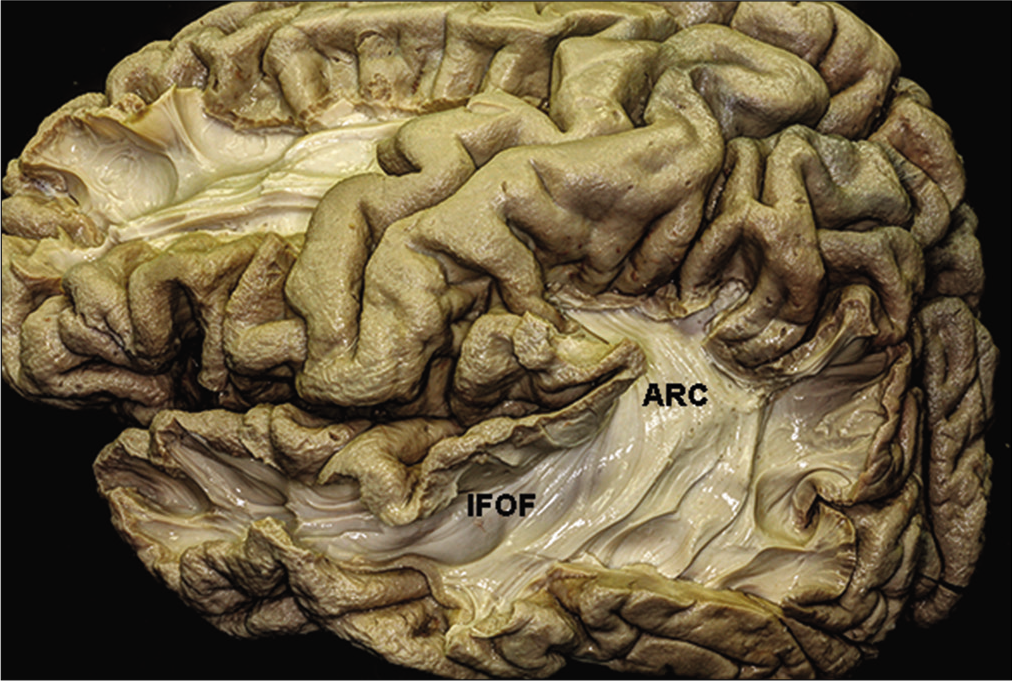
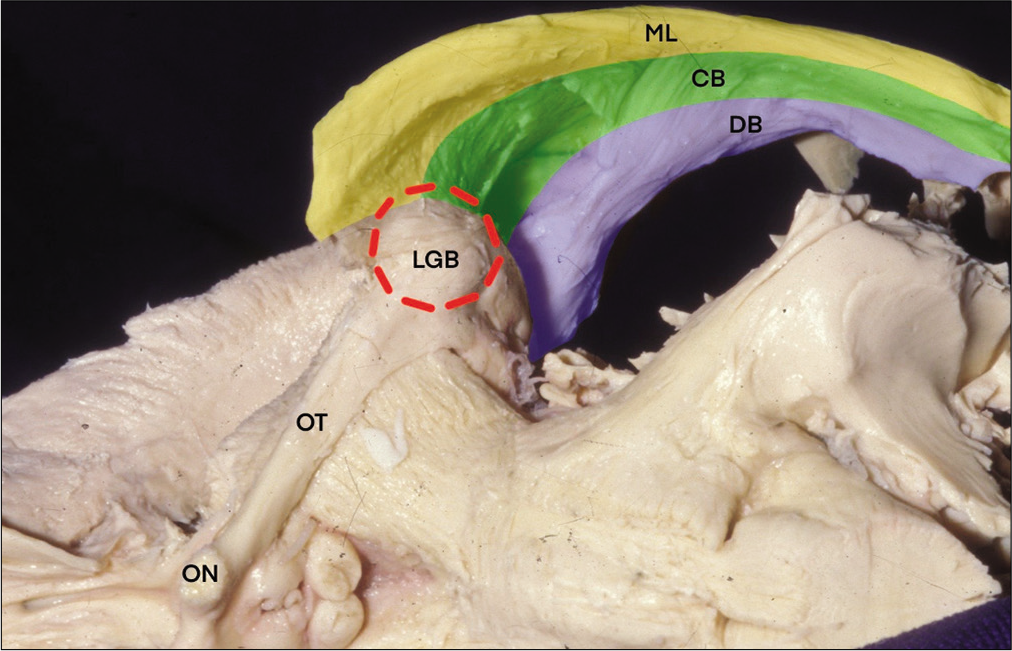
The optic radiations originate from the lateral geniculate body (LGB) and extend into the calcarine fissure in the occipital lobe [
The anterior fiber bundle or Meyer’s loop
The anterior bundle of the optic radiations, in the first part of its trajectory, courses along the upper half of the medial aspect of the temporal horn. As the fiber bundle reaches a more anterior point, it changes the position to course along the roof of the temporal horn beneath the lentiform nucleus and above the caudate nucleus until it reaches the anterior tip of the temporal horn. The fiber bundle extends an average of 2 mm anterior to the tip of the temporal horn[
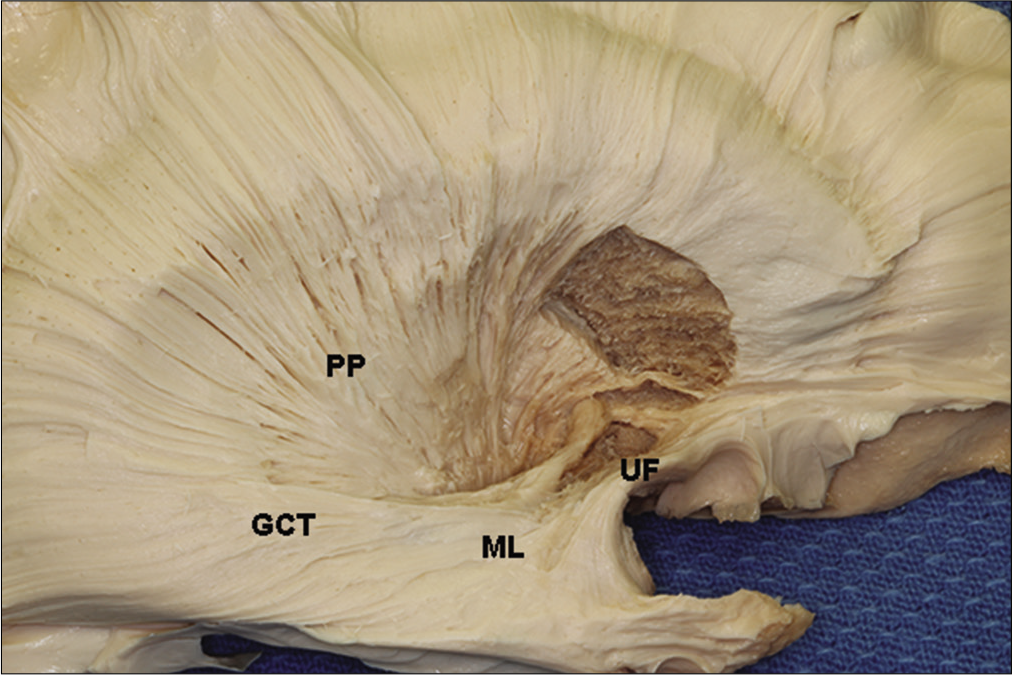
At the edge of the temporal lobe, the optic radiations are located just behind the fibers that connect the frontal and temporal lobes, a fiber tract known as uncinate fasciculus, and above the amygdala. After reaching this extreme point, the course of the fibers does a posterior loop in a more lateral trajectory to reach the occipital cortex. This curvature is known as Meyer’s loop. As the fibers run backward, they again course along the roof of the temporal horn, but now in a more lateral trajectory extending along the upper half of the lateral wall of the temporal horn, being considered an important structure on the wall of the lateral ventricle.
While coursing on the wall of the lateral ventricle, the anterior fiber bundle is located beneath the central fiber bundle. This occurs because, in the anterior portion of the temporal lobe, the anterior fiber bundle is located lateral to the central fiber bundle. However, as the fibers run posteriorly, they curve down to beneath the central fiber bundle to reach the lower lip of the calcarine sulcus.[
The central fiber bundle
The central bundle of the optic radiations emerges from the LGB and contains the largest number of fibers. In the first part of its trajectory, the central fiber bundle runs laterally along the roof of the temporal horn. It, then, curves slightly backward in a way that the anterior fibers of the central bundle may be mistaken for the posterior fibers of the anterior bundle. This partial loop occurs in the upper half of the lateral wall of the temporal horn. The fibers of the anterior bundle merge with the fibers of the lateral wall of the temporal horn to form the sagittal stratum, after which they continue in a retrograde fashion to reach the occipital pole.
The posterior fiber bundle
This fiber bundle courses posteriorly until it reaches the calcarine fissure. These fibers pass directly to the occipital pole, where they meet the fibers of the central and anterior bundles. In this trajectory, the fibers of the sagittal stratum cover the lateral wall of the atrial portion of the ventricle. Altogether they form the sagittal stratum, where the anterior fiber bundle comprises the lower part and reaches the upper lip of the calcarine fissure.
The optic radiations and the temporal horn
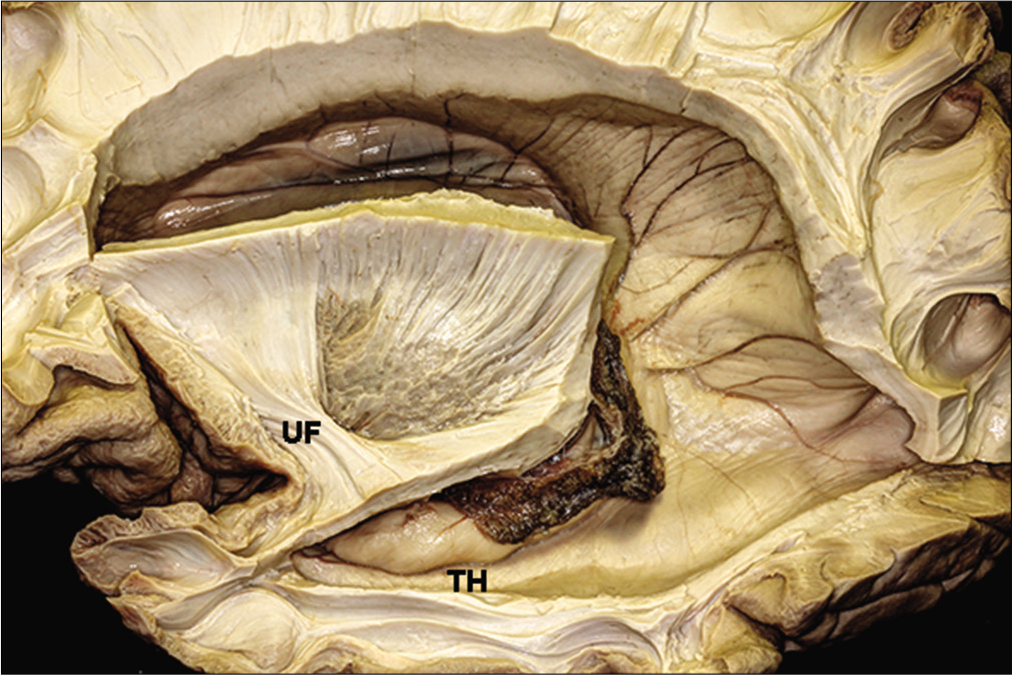
The temporal horn is closely related to the optic radiations [
The entire superior lateral wall of the temporal horn is covered by the optic radiations, while the inferior medial wall of the temporal horn is free from the optic radiations, except at the level of the LGB.[
The optic radiations and the insula and uncal recess
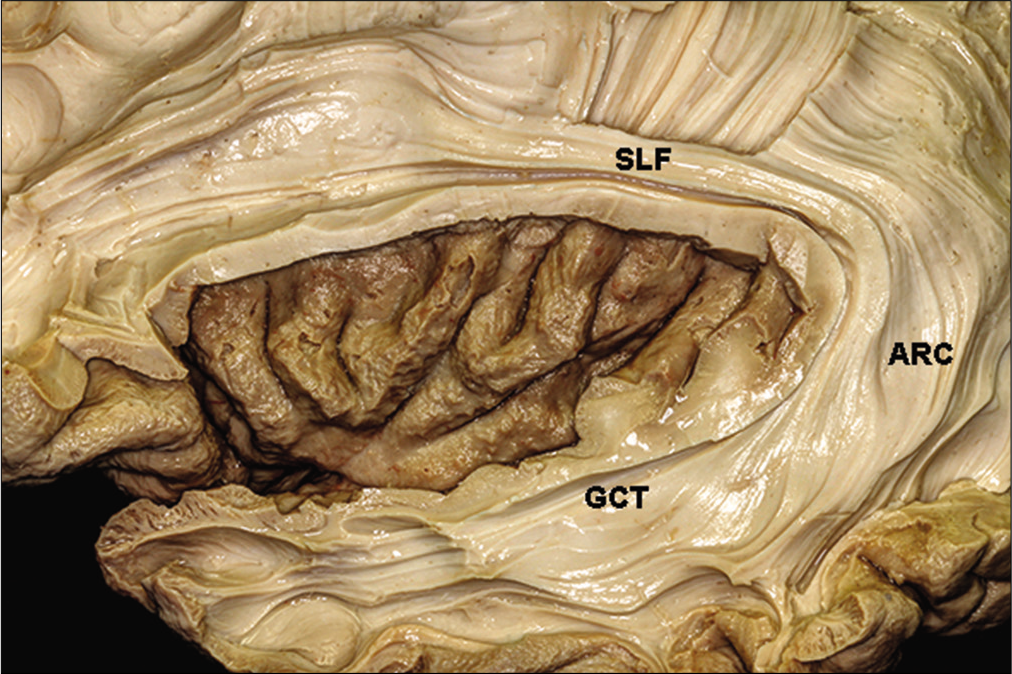
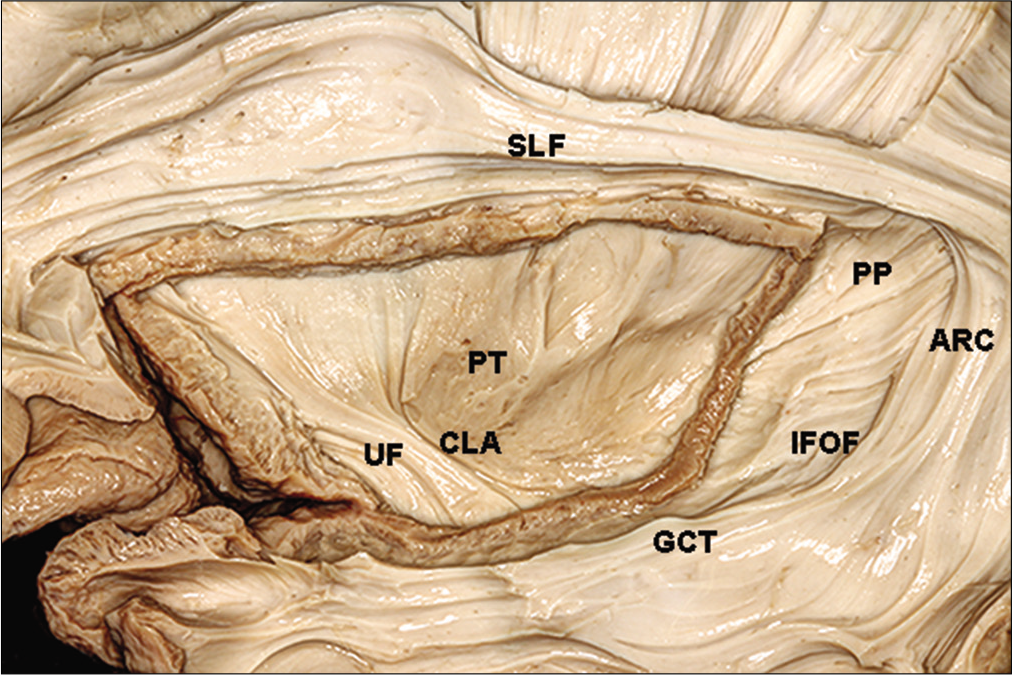
The insula is complexly related to the optic radiations. The insular cortex can be seen only if the sylvian fissure is opened. It is an island of cortical tissue completely covered by the frontal operculum and by the superior temporal gyrus. Removal of the insular cortex uncovers the arcuate fibers, which connect the frontal, parietal, temporal, and occipital lobes. Dissection of the fibers of the extreme capsule reveals the claustrum.
The claustrum is surrounded medially by the fibers of the external capsule, which is located between the claustrum and the lenticular nucleus.[
Interruption of the optic radiations during resection for lateral access to the temporal horn is shown in
Neurophysiology of the visual pathways and the optic tract
The retina is formed by a variety of cells, with rods and cones being the first neurons in this complex network of fibers conveying visual information to the occipital cortex. The light must pass through all layers of the retina to reach its external part, where the photoreceptors (rods and cones) are located. The photoreceptors are connected by synapses with the bipolar cells and the bipolar cells with the ganglion cells. Along the sulcus rectus, the optic nerve (CN II) is formed exclusively by unmyelinated axons of the ganglion cells that originate from the retina and reach the brain by passing through the optic canal together with the ophthalmic artery. The optic nerves (from both eyes) condense in the optic chiasm. The fibers from the nasal retina (temporal visual field) change sides, while the fibers from the temporal retina (nasal vision field) remain on the same side.
The optic tract connects the optic chiasm and the LGB – the connecting point from which the optic radiations will emerge. The LGB also connects to the superior colliculus, from where the visual fibers extend to the pulvinar and then to Brodmann’s areas 18 and 19 and to the midbrain, generating a number of eye reflexes coordinated by the Edinger–Westphal nucleus. The optic radiations convey visual information to the occipital cortex, more specifically to Brodmann’s area 17, where visual consciousness arises. Its microanatomical pathway is very complex and has been described previously.[
DISCUSSION
For neurosurgeons, the most important relationships are those between the temporal lobe anatomy and the optic radiations. Of paramount, importance is the knowledge of the relationship between the tip of the temporal horn and the most anterior fibers of Meyer’s loop [
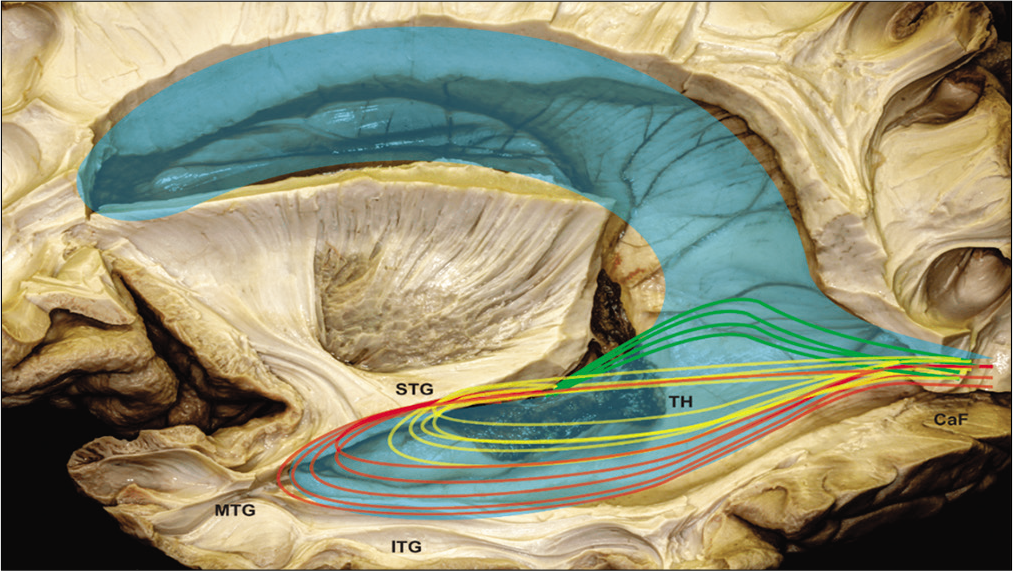
Figure 8:
Illustration of optical radiation superimposed on the dissected anatomical image. STG: Superior temporal gyrs, MTG: middle temporal gyrus, ITG: inferior temporal gyrus, TH: temporal horn, Caf: calcarine fissure, Green trace: Meyer’s loop, Yellow trace: central bundle, and Red trace: dorsal bundle.
Surgical approaches and the optic radiations
Standard temporal lobectomy or modified temporal lobectomy using Spencer’s technique[
In a microanatomical analysis, access to medial temporal lobe structures through the sylvian fissure have the potential to preserve the integrity of the optic radiations during surgery.[
CONCLUSION
The optic radiations and their relationship to other structures in the white matter must be considered when planning the resection of medial temporal lobe structures. To avoid optic radiations, the surgical approach arsenal consists of three trajectories: transsylvian, subtemporal, and paramedian supracerebellar transtentorial. All these approaches carry risks and benefits. For better neurosurgical planning, not only the cortex and the target structures of the surgery must be considered but also what lies in the way, in this case, the white fibers of the optic tract and the optic radiations.
Although not commonly used in epilepsy surgery to avoid injury to the optic radiations, intraoperative cortical and subcortical mapping of the optic radiations have been increasingly used in awake surgery to resect tumors in eloquent areas.[
The integration of anatomical knowledge is of fundamental importance for adequate neurosurgical planning, to avoid as much as possible surgical damage to optical radiation. In this sense, more recently, we managed to envision neurosurgical techniques that associate anatomical knowledge, magnetic resonance images with the combination of intraoperative neuronavigation.[
The association of technological innovation using magnetic resonance images with neuronavigation applied to microscopic imaging can significantly reduce injuries secondary to optical radiation, as demonstrated in some series studied and compared with documented visual campimetry.[
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Catani M, Howard RJ, Pajevic S, Jones DK. Virtual in vivo interactive dissection of white matter fasciculi in the human brain. Neuroimage. 2002. 17: 77-94
2. Choi C, Rubino PA, Fernandez-Miranda JC, Abe H, Rhoton AL. Meyer’s loop and the optic radiations in the transsylvian approach to the mediobasal temporal lobe. Neurosurgery. 2006. 59: 228-35
3. Cui Z, Ling Z, Pan L, Song H, Chen X, Shi W. Optic radiation mapping reduces the risk of visual field deficits in anterior temporal lobe resection. Int J Clin Exp Med. 2015. 8: 14283-95
4. de Castro I, de Christoph DH, dos Santos DP, Landeiro JÁ. Internal structure of the cerebral hemispheres: an introduction of fiber dissection technique. Arq Neuropsiquiatr. 2005. 63: 252-8
5. de Oliveira JG, Parraga RG, Chaddad-Neto F, Ribas GC, de Oliveira EP. Supracerebellar transtentorial approach-resection of the tentorium instead of an opening-to provide broad exposure of the mediobasal temporal lobe: Anatomical aspects and surgical applications: clinical article. J Neurosurg. 2012. 116: 764-72
6. Gras-Combe G, Moritz-Gasser S, Herbet G, Duffau H. Intraoperative subcortical electrical mapping of optic radiations in awake surgery for glioma involving visual pathways. J Neurosurg. 2012. 117: 466-73
7. Kawashima M, Li X, Rhoton AL, Ulm AJ, Oka H, Fujii K. Surgical approaches to the atrium of the lateral ventricle: Microsurgical anatomy. Surg Neurol. 2006. 65: 436-45
8. Klingler J. Erleichterung der makroskopischen preparation des gehirns durch den Gefrierprozess. Schweiz Arch Neurol Psychiatr. 1935. 36: 247-56
9. Niemeyer P.editors. The transventricular amygdala-hippocampectomy in temporal lobe epilepsy. Temporal Lobe Epilepsy. Springfield, Ill: Charles C Thomas; 1958. p. 461-82
10. Pathak-Ray V, Ray A, Walters R, Hatfield R. Detection of visual field defects in patients after anterior temporal lobectomy for mesial temporal sclerosis-establishing eligibility to drive. Eye. 2002. 16: 744-8
11. Peltier J, Travers N, Destrieux C, Velut S. Optic radiations: A microsurgical anatomical study. J Neurosurg. 2006. 105: 294-300
12. Peuskens D, van Loon J, Van Calenbergh F, van den Bergh R, Goffin J, Plets C. Anatomy of the anterior temporal lobe and the frontotemporal region demonstrated by fiber dissection. Neurosurgery. 2004. 55: 1174-84
13. Rubino PA, Rhoton AL, Tong X, Oliveira E. Three-dimensional relationships of the optic radiation. Neurosurgery. 2005. 57: 219-27
14. Sincoff EH, Tan Y, Abdulrauf SI. White matter fiber dissection of the optic radiations of the temporal lobe and implications for surgical approaches to the temporal horn. J Neurosurg. 2004. 101: 739-46
15. Spencer DD, Spencer SS, Mattson RH, Williamson PD, Novelly RA. Access to the posterior medial temporal lobe structures in the surgical treatment of temporal lobe epilepsy. Neurosurgery. 1984. 15: 667-71
16. Ture U, Harput MV, Kaya AH, Baimedi P, Firat Z, Ture H. The paramedian supracerebellar-transtentorial approach to the entire length of the mediobasal temporal region: an anatomical and clinical study. Laboratory investigation. J Neurosurg. 2012. 116: 773-91
17. Ture U, Yasargil MG, Friedman AH, Al-Mefty O. Fiber dissection technique: Lateral aspect of the brain. Neurosurgery. 2000. 47: 417-26
18. Winston GP, Daga P, White MJ, Micallef C, Miserocchi A, Mancini L. Preventing visual field deficits from neurosurgery. Neurology. 2014. 83: 604-11
19. Winston GP, Mancini L, Stretton J, Ashmore J, Symms MR, Duncan JS. Diffusion tensor imaging tractography of the optic radiation for epilepsy surgical planning: A comparison of two methods. Epilepsy Res. 2011. 97: 124-32
20. Yasargil MG, Ture U, Yasargil DC. Impact of temporal lobe surgery. J Neurosurg. 2004. 101: 725-38
21. Yasargil MG, Wieser HG, Valavanis A, von Ammon K, Roth P. Surgery and results of selective amygdala-hippocampectomy in one hundred patients with nonlesional limbic epilepsy. Neurosurg Clin N Am. 1993. 4: 243-61
22. Yeni SN, Tanriover N, Uyanik O, Ulu MO, Ozkara C, Karaagac N. Visual field defects in selective amygdalohippocampectomy for hippocampal sclerosis: The fate of Meyer’s loop during the transsylvian approach to the temporal horn. Neurosurgery. 2008. 63: 507-13