- Department of Neurosurgery, Asklepieion Hospital of Voula, Voula, Greece
- Department of Neurosurgery, University of Athens Medical School, “Evangelismos” General Hospital, Athens, Greece
- Department of Neurosurgery, University of Thessaloniki Medical School, “AHEPA” University Hospital, Thessaloniki, Greece
- Department of Neurosurgery, Children's Hospital “Agia Sofia”, Athens, Greece
- Department of Neurosurgery, Kings College Hospital NHS Foundation Trust, London, UK
Correspondence Address:
Blionas Alexandros
Department of Neurosurgery, Children's Hospital “Agia Sofia”, Athens, Greece
DOI:10.4103/sni.sni_188_17
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Blionas Alexandros, Giakoumettis Dimitrios, Antoniades Elias, Drosos Evangelos, Mitsios Andreas, Plakas Sotirios, Sfakianos Georgios, Themistocleous S. Marios. Aplasia cutis congenita: Two case reports and discussion of the literature. 09-Nov-2017;8:273
How to cite this URL: Blionas Alexandros, Giakoumettis Dimitrios, Antoniades Elias, Drosos Evangelos, Mitsios Andreas, Plakas Sotirios, Sfakianos Georgios, Themistocleous S. Marios. Aplasia cutis congenita: Two case reports and discussion of the literature. 09-Nov-2017;8:273. Available from: http://surgicalneurologyint.com/surgicalint-articles/aplasia-cutis-congenita-two-case-reports-and-discussion-of-the-literature/
Abstract
Background:Aplasia cutis congenita (ACC) is a part of a heterogeneous group of conditions characterized by the congenital absence of epidermis, dermis, and in some cases, subcutaneous tissues or bone usually involving the scalp vertex. There is an estimated incidence of 3 in 10,000 births resulting in a total number of 500 reported cases to date. The lesions may occur on every body surface although localized scalp lesions form the most frequent pattern (70%). Complete aplasia involving bone defects occurs in approximately 20% of cases. ACC can occur as an isolated defect or can be associated with a number of other congenital anomalies such as limb anomalies or embryologic malformations. In patients with large scalp and skull defects, there is increased risk of infection and bleeding along with increased mortality and therefore prompt and effective management is advised.
Case Description:We describe two cases of ACC, involving a 4 × 3 cm defect managed conservatively and a larger 10 × 5 cm defect managed surgically with the use of a temporo-occipital scalp flap. Both cases had an excellent outcome.
Conclusions:Multiple treatment regimens exist for ACC, but there is no consensus on treatment strategies. Conservative treatment has been described and advocated, but many authors have emphasized the disadvantages of this treatment modality. Decision between conservative and surgical management must be individualized according to lesion size and location.
Keywords: Aplasia cutis congenita, congenital anomalies, cranial reconstruction, scalp reconstruction
INTRODUCTION
Aplasia cutis congenita (ACC) is a heterogenous group of disorders reported historically by Cordon in 1767, and characterized by the absence of epidermis, dermis, and occasionally subcutaneous tissues or even bone tissue, involving multiple possible body locations. The most common lesion location is the scalp (70%);[
ACC holds an estimated incidence of about 3 in 10,000 births and there has been a total of approximately 500 reported cases in the literature.[
Pathophysiology of ACC is not well studied and its exact pathogenesis is unknown.[
Chromosomal abnormalities,[ Amniotic irregularities[ Intrauterine complications, such as vascular accidents or infection[ Teratogens: such as misoprostol, benzodiazepines, valproic acid cocaine, methotrexate, ACE inhibitors, methimasol.[
The main pathophysiologic hypothesis about ACC is that the mechanism behind it lies in tension-induced disruption of the overlying skin occurring at 10–15 weeks of gestation when rapid brain growth occurs along with hair direction and patterning.[
CASE DESCRIPTIONS
The following cases are categorized as group 1 ACC and involve patients with multiple scalp bullous lesions, with both skin and bone layer defects. We will describe both cases and discuss presentation, prognosis, and management strategies.
First case
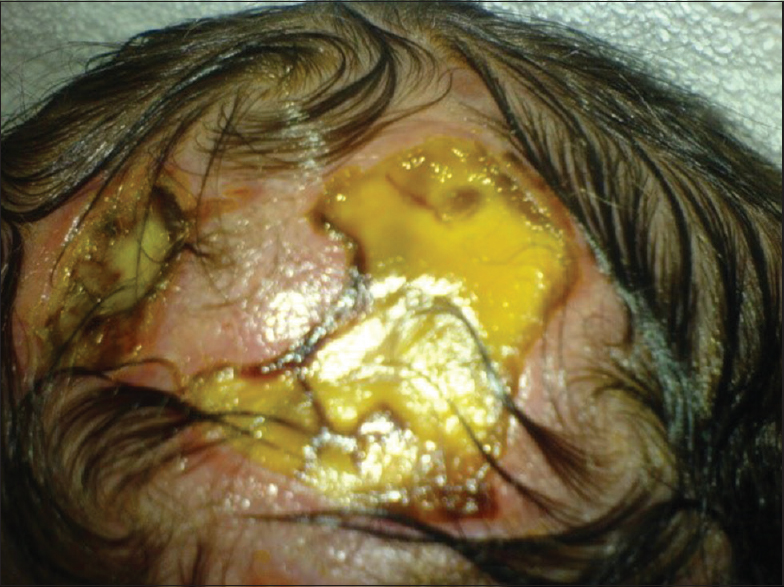
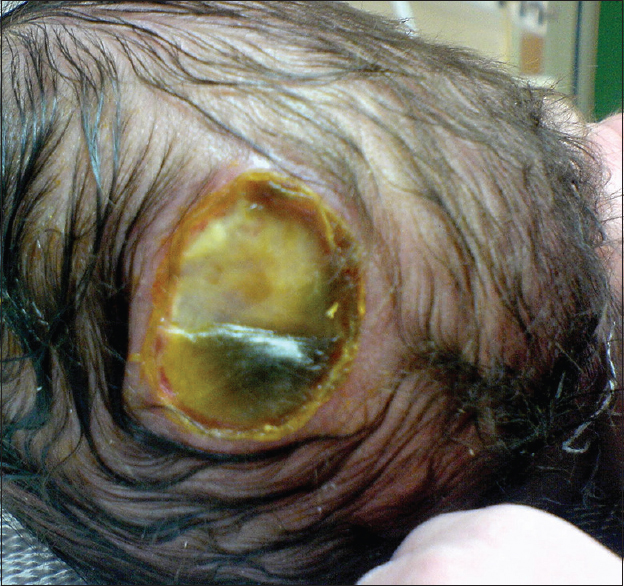
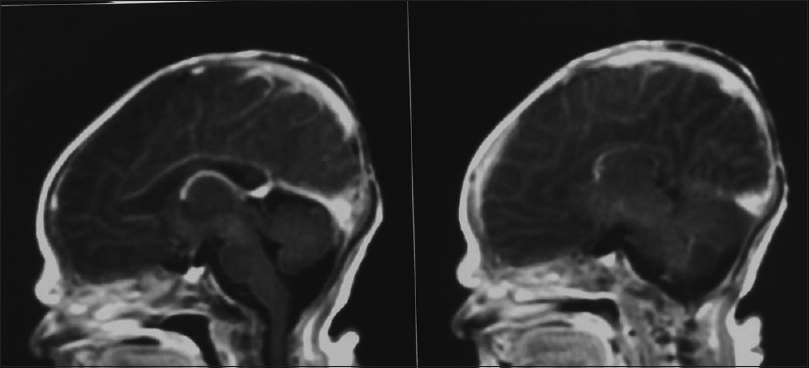
Our first patient was a 3300 g white boy of 37 weeks of gestation. Pregnancy, labor, and delivery were without any mishaps. From the obstetrical record, we were informed that the mother was treated for hypothyroidism with T4 and also with Ritodrine (Utopar®) due to uterine contractions. No abnormal family history was reported. At birth, the boy was found to have three sizable bullae on his scalp. There was a large round-shaped occipital defect at the vertex with dimensions of 4 × 3 cm and two lesser ones at the frontal vertex with dimensions of 1 × 2 and 1 × 1 cm [Figures
Second case
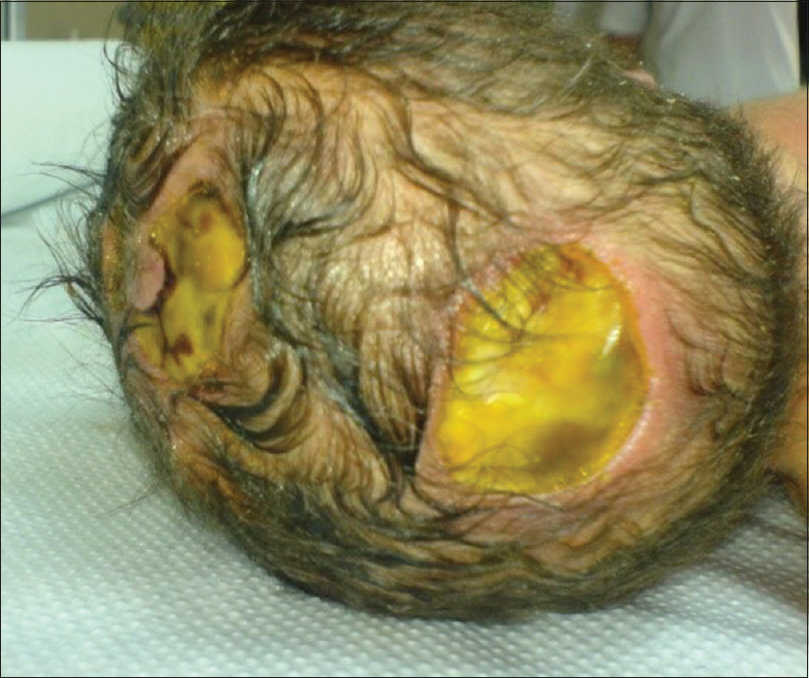
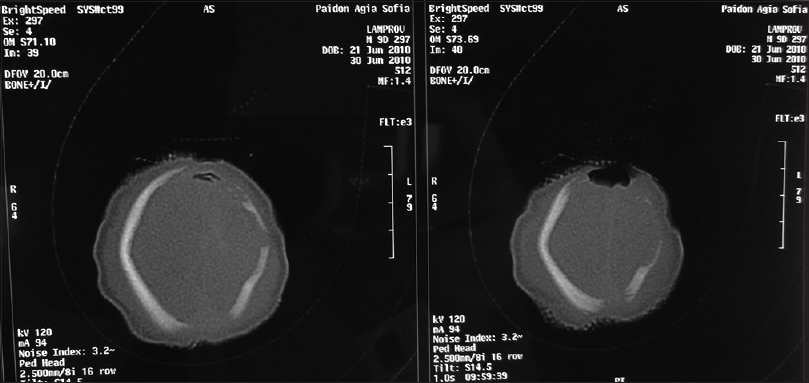
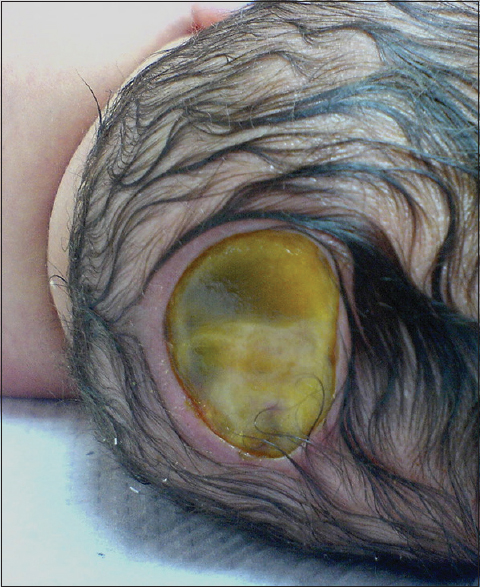
Our second patient was a 3660 g white boy of 42 weeks of gestation. Pregnancy, labor, and delivery were without any mishaps. The obstetrical record included history of induced delivery. The medical record revealed a family history of ACC involving the patient's father and siblings. At birth, the patient had an extensive round-shaped hemorrhagic bullae with dimensions of 10 × 5 cm above the fronto-occipital regions of the scalp [Figures
DISCUSSION
In the majority of cases (70%) ACC manifests as a solitary defect of the scalp, but occasionally it may present with multiple lesions such as in our first case. Although lesions are noninflammatory and well demarcated, there is great controversy concerning treatment of ACC and there has been a great scientific interest due to the extremely high mortality figures that range from 20 to 55%.[
The management of scalp ACC is controversial. Treatment may be either conservative or operative, and there is no consensus or guidelines on treatment strategy. Choosing between conservative and operative modalities is challenging and should be individualized.[
Conservative management consists of regular wound cleansing and application of dressings along with the use of systemic antibiotics.[
Surgical management, on the contrary, includes various procedures. Standard surgical care includes primary wound closure, skin grafting (autologous or allografts),[
CONCLUSION
In conclusion, we strongly believe that a conservative approach minimizes complications, avoids unwanted operative sequelae, and takes full advantage of the innate rapid regeneration ability of the newborn.[
In our specific cases, we utilized a conservative approach in Case 1, which involved a smaller defect (1 × 2 cm), whereas for the larger defect of Case 2 (10 × 5 cm), a surgical approach with the application of a single scalp flap was utilized. Results were satisfying in both cases, with complete wound closure. Taking into account both our own experience and the literature, we strongly believe that utilizing a size threshold for deciding between conservative or surgical approach along with meticulous sterile dressings and wound care is the optimal management strategy for ACC. The optimal size threshold and specific treatment guidelines remain yet to be determined. The literature complete lacks any precise guidelines or suggestions on the subject and further research is required. Therefore, it must be noted that due to the lack of treatment algorithms and guidelines on ACC management and the need for individualized treatment decisions, it is important for such cases to be managed in centers specialized in pediatric neurosurgery with sufficient experience and expertise.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Abbott R, Cutting CB, Wisoff JH, Thorne CH, Epstein FJ. Aplasia cutis congenita of the scalp: Issues in its management. Pediatr Neurosurg 1991. 1992. 17: 182-4
2. Attalla MF, el-Sayed AM. Scalp aplasia cutis congenita: Closure by the L-shaped flap. Childs Nerv Syst. 1992. 8: 287-8
3. Azad S, Falder S, Harrison J, Graham K. An adherent dressing for aplasia cutis congenita. Br J Plast Surg. 2005. 58: 1159-61
4. Başterzi Y1, Baǧdatoǧlu C, Sari A, Demirkan F. Aplasia cutis congenita of the scalp and calvarium. J Craniofac Surg. 2007. 18: 427-9
5. Beekmans SJA, Haumann TJ, Vandertop WP, Mulder JW. [Aplasia cutis congenita in 4 infants]. Ned Tijdschr Geneeskd. 2002. 146: 1842-5
6. Bharti G, Groves L, David LR, Sanger C, Argenta LC. Aplasia Cutis Congenita. J Craniofac Surg. 2011. 22: 159-65
7. Brzezinski P, Pinteala T, Chiriac AE, Foia L, Chiriac A. Aplasia cutis congenita of the scalp—what are the steps to be followed? Case report and review of the literature. An Bras Dermatol. 2015. 90: 100-3
8. Caksen H, Kurtoglu S. Our experience with aplasia cutis congenita. J Dermatol. 2002. 29: 376-9
9. Choi MSS, Choi JH, Ki SH, Jun YH. Aplasia cutis congenita associated with aplasia of the superficial temporal artery. J Craniofac Surg. 2016. 27: 1065-7
10. Donati V, Arena S, Capilli G, Carrera G, Ciralli F, Liberatore A. Reparation of a severe case of aplasia cutis congenita with engineered skin. Biol Neonate. 2001. 80: 273-6
11. Duan X, Yang G, Yu D, Yu C, Wang B, Guo Y. Aplasia cutis congenita: A case report and literature review. Exp Ther Med. 2015. 10: 1893-95
12. Dutra LB, Pereira MD, Kreniski TM, Zanon N, Cavalheiro S, Ferreira LM. Aplasia cutis congenita. J Craniofac Surg. 2009. 20: 1288-92
13. Frieden IJ. Aplasia cutis congenita: A clinical review and proposal for classification. J Am Acad Dermatol. 1986. 14: 646-60
14. Fröjd V, Maltese G, Kölby L, Tarnow P. Conservative Healing of an 11 × 9-cm Aplasia cutis congenita of the scalp with bone defect. J Neurol Surg Reports. 2014. 75: e220-3
15. Guinard D, Lebeau J, Moutet F, Raphael B. [Congenital cutaneous aplasia. Apropos of 3 new cases located in the vertex]. Ann Chir Plast Esthet. 1993. 38: 513-9
16. Hadad I, Meara JG, Rogers-Vizena CR. A novel local autologous bone graft donor site after scalp tissue expansion in aplasia cutis congenita. J Craniofac Surg. 2016. 27: 904-7
17. Harvey G, Solanki NS, Anderson PJ, Carney B, Snell BJ. Management of aplasia cutis congenita of the scalp. J Craniofac Surg. 2012. 23: 1662-4
18. Iwayama H, Hosono H, Yamamoto H, Oshiro M, Ueda N. Aplasia cutis congenita with skull defect in a monozygotic twin after exposure to methimazole in utero. Birth Defects Res. Part A Clin Mol Teratol. 2007. 79: 680-4
19. Johnson R, Offiah A, Cohen MC. Fatal superior sagittal sinus hemorrhage as a complication of aplasia cutis congenita: A case report and literature review. Forensic Sci Med Pathol. 2015. 11: 243-8
20. Kalb RE, Grossman ME. The association of aplasia cutis congenita with therapy of maternal thyroid disease. Pediatr Dermatol. 1986. 3: 327-30
21. Karg E, Bereg E, Gaspar L, Katona M, Turi S. Aplasia cutis congenita after methimazole exposure in utero. Pediatr Dermatol. 2004. 21: 491-4
22. Kruk-Jeromin J, Janik J, Rykała J. Aplasia cutis congenita of the scalp. Report of 16 cases. Dermatol Surg. 1998. 24: 549-53
23. Lahiri A, Nishikawa H. A nonadherent dressing for aplasia cutis congenita. J Plast Reconstr Aesthetic Surg. 2006. 59: 781-2
24. Leboucq N, Montoya y Mártínez P, Montoya-Vigo F, Catan P. Aplasia cutis congenita of the scalp with large underlying skull defect: A case report. Neuroradiology. 1994. 36: 480-2
25. Levine SM, Reformat DD, Thorne CH. Cutis aplasia: Perioperative management and case report. Am J Crit Care. 2012. 21: 212-5
26. Maillet-Declerck M, Vinchon M, Guerreschi P, Pasquesoone L, Dhellemmes P, Duquennoy-Martinot V. Aplasia cutis congenita: Review of 29 cases and proposal of a therapeutic strategy. Eur J Pediatr Surg. 2012. 23: 89-93
27. Marble M, Guillen Sacoto MJ, Chikarmane R, Gargiulo D, Juusola J. Missense variant in UBA2 associated with aplasia cutis congenita, duane anomaly, hip dysplasia and other anomalies: A possible new disorder involving the SUMOylation pathway. Am J Med Genet Part A. 2017. 173: 758-61
28. Marcovici I. Aplasia cutis congenita presenting as vacuum-extractor-related trauma. Int J Gynecol Obstet. 2015. 129: 267-8
29. Marneros AG. BMS1 is mutated in aplasia cutis congenita. PLoS Genet. 2013. 9: e1003573-
30. Mesrati H, Amouri M, Chaaben H, Masmoudi A, Boudaya S, Turki H. Aplasia cutis congenita: Report of 22 cases. Int J Dermatol. 2015. 54: 1370-5
31. Moros Peña M, Labay Matías M, Valle Sánchez F, Valero Adán T, Martín-Calama Valero J, Muñoz Albillos M. [Aplasia cutis congenita in a newborn: Etiopathogenic review and diagnostic approach]. An Esp Pediatr. 2000. 52: 453-6
32. O’Neill JK, Carter M, Warr RP. Aplasia cutis congenita. A case of scalp defect repair using two opposing bipedicled local flaps. J Plast Reconstr Aesthetic Surg. 2010. 63: e242-4
33. Orgun D, Horiguchi M, Hayashi A, Shimoji K, Arai H, Mizuno H. Conservative treatment of large aplasia cutis congenita of the scalp with bone defect with basic fibroblast growth factor application. J Craniofac Surg. 2016. p. 1-
34. Ploplys EA, Muzaffar AR, Gruss JS, Ellenbogen RG. Early composite cranioplasty in infants with severe aplasia cutis congenita: A report of two cases. Cleft Palate-Craniofacial J. 2005. 42: 442-7
35. Prager W, Scholz S, Rompel R. Aplasia cutis congenita in two siblings. Eur J Dermatol. 2002. 12: 228-30
36. Renfree KJ, Dell PC. Distal limb defects and aplasia cutis: Adams–Oliver Syndrome. J Hand Surg Am. 2016. 41: e207-10
37. Rhee ST, Colville C, Buchman SR, Muraszko K. Complete osseous regeneration of a large skull defect in a patient with cutis aplasia: A conservative approach. J Craniofac Surg. 2002. 13: 497-500
38. Ribuffo D, Costantini M, Gullo P, Houseman ND, Taylor GI. Aplasia cutis congenita of the scalp, the skull, and the dura. Scand J Plast Reconstr Surg Hand Surg. 2003. 37: 176-80
39. Rodríguez-García C, González-Hernández S, Hernández-Martín A, Pérez-Robayna N, Sánchez R, Torrelo A. Aplasia cutis congenita and other anomalies associated with methimazole exposure during pregnancy. Pediatr Dermatol. 2011. 28: 743-5
40. Sachs C, Tebacher-Alt M, Mark M, Cribier B, Lipsker D. Aplasie cutanée congénitale et antithyroïdiens de synthèse au cours de la grossesse: Série de cas et revue de la littérature. Ann Dermatol Venereol. 2016. 143: 423-35
41. Santos de Oliveira R, Barros Jucá CE, Lopes Lins-Neto A, Aparecida do Carmo Rego M, Farina J, Machado HR. Aplasia cutis congenita of the scalp: Is there a better treatment strategy?. Child's Nerv Syst. 2006. 22: 1072-9
42. Sargent LA. Aplasia cutis congenita of the scalp. J Pediatr Surg. 1990. 25: 1211-3
43. Schnabl SM, Horch RE, Ganslandt O, Schroth M, Dragu A, Bach AD. Aplasia cutis congenita – plastic reconstruction of three scalp and skull defects with two opposed scalp rotation flaps and split thickness skin grafting. Neuropediatrics. 2009. 40: 134-6
44. Shivakumar SK, Dwarakanath S, Swaroop G, Venkataramana NK. Aplasia cutis congenita of the scalp: Therapeutic modalities. Neurol India. 2006. 54: 312-3
45. Silberstein E, Pagkalos VA, Landau D, Berezovsky AB, Krieger Y, Shoham Y. Aplasia cutis congenita. Plast Reconstr Surg. 2014. 134: 766e-74e
46. Šimić D, Prohić A, Puizina Ivić N, Zeljko Penavić J, Tomić T. Aplasia cutis congenita in a newborn child associated with two fetus Papyraceous. Acta Dermatovenerol Croat. 2015. 23: 293-7
47. Singh M, Bui CJ, St-Hilaire H. Reconstruction of complex aplasia cutis congenita. J Craniofac Surg. 2012. 23: e88-e90
48. Six EG, Kelly DL. Conservative management of aplasia cutis congenita: Case report. Neurosurgery. 1981. 8: 233-5
49. Steinbacher J, Rath T, Tzou C-H. Aplasia cutis congenita. Handchirurgie Mikrochirurgie Plast Chir. 2016. 48: 239-43
50. Suárez O, López-Gutiérrez JC, Andrés A, Barrena S, Encinas JL, Luis A. [Aplasia cutis congenita: Surgical treatment and results in 36 cases]. Cir Pediatr. 2007. 20: 151-5
51. Sugiura T, Kouwaki M, Kiyosawa S, Sasada Y, Maeda M, Goto K. A case of systemic aplasia cutis congenita: A newly recognized syndrome?. Eur J Pediatr. 2008. 167: 409-13
52. Theile RJ, Lanigan MW, McDermant GR. Reconstruction of aplasia cutis congenita of the scalp by split rib cranioplasty and a free latissimus dorsi muscle flap in a nine month old infant. Br J Plast Surg. 1995. 48: 507-10
53. Trevizo Ortiz L, Ruiz-Maldonado R, Tamayo SL. [Aplasia cutis congenita]. Bol Med Hosp Infant Mex. 1978. 35: 333-42
54. Vanamo K, Härmä M. The Shoelace method in congenital aplasia of the scalp and skull. Eur J Pediatr Surg. 2005. 15: 425-7
55. Vogt T, Stolz W, Landthaler M. Aplasia cutis congenita after exposure to methimazole: A causal relationship?. Br J Dermatol. 1995. 133: 994-6
56. Wexler A, Harris M, Lesavoy M. Conservative treatment of cutis aplasia. Plast Reconstr Surg. 1990. 86: 1066-71
57. Yang JY, Yang WG. Large scalp and skull defect in aplasia cutis congenita. Br J Plast Surg. 2000. 53: 619-22
58. Yilmaz S, Apaydin I, Yenidünya O, Adanali G, Gültan S. Conservative management of aplasia cutis congenita. Dermatol Surg. 1997. 23: 402-3
59. Zhou J, Zheng L, Tao W. Systemic aplasia cutis congenita: A case report and review of the literature. Pathol Res Pract. 2010. 206: 504-7