- Department of Biology Northern Arizona University, Flagstaff, Arizona, United States.
- Department of Mechanical Engineering Northern Arizona University, Flagstaff, Arizona, United States.
- Center for Materials Interfaces in Research and Applications, Northern Arizona University, Flagstaff, Arizona, United States.
- Department of Neurosurgery Research, Barrow Neurological Institute, Phoenix, Arizona, United States.
Correspondence Address:
Timothy Andrew Becker, Department of Mechanical Engineering, Northern Arizona University, Flagstaff, Arizona, United States.
DOI:10.25259/SNI_163_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: April Huckleberry1, William Merritt2, Trevor Cotter3, Christopher Settanni2, Mark C. Preul4, Andrew F. Ducruet4, Timothy Andrew Becker2. Application of a rabbit-elastase aneurysm model for preliminary histology assessment of the PPODA-QT liquid embolic. 06-Jul-2021;12:330
How to cite this URL: April Huckleberry1, William Merritt2, Trevor Cotter3, Christopher Settanni2, Mark C. Preul4, Andrew F. Ducruet4, Timothy Andrew Becker2. Application of a rabbit-elastase aneurysm model for preliminary histology assessment of the PPODA-QT liquid embolic. 06-Jul-2021;12:330. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=10948
Abstract
Background: PPODA-QT is a novel liquid embolic under development for the treatment of cerebral aneurysms. We sought to test the rabbit-elastase aneurysm model to evaluate the tissue response following PPODA-QT embolization.
Methods: Experimental elastase-induced aneurysms were created in fourteen New Zealand White Rabbits. Eight animals were used for aneurysm model and endovascular embolization technique development. Six PPODA-QT-treated animals were enrolled in the study. Control and aneurysm tissues were harvested at acute (n = 2), 1-month (n = 2), and 3-month (n = 2) timepoints and the tissues were prepared for histology assessment.
Results: All fourteen rabbit-elastase aneurysms resulted in small and medium aneurysm heights (d: n) ratios. Histological evaluation of four aneurysms, treated with PPODA-QT, demonstrated reorganization of aneurysm wall elastin into a smooth muscle layer, and observed as early as the 1-month survival timepoint. At the aneurysm neck, a homogenous neointimal layer (200–300 μm) formed at the PPODA-QT interface, sealing off the parent vessel from the aneurysm dome. No adverse immune response was evident at 1- and 3-month survival timepoints.
Conclusion: PPODA-QT successfully embolized the treated aneurysms. Following PPODA-QT embolization, neointimal tissue growth and remodeling were noted with minimal immunological response. The experimental aneurysms created in rabbits were uniformly small with inconsistent neck morphology. Further testing of PPODA-QT will be conducted in larger aneurysm models for device delivery optimization and aneurysm healing assessment before human clinical investigation.
Keywords: Aneurysm, Device, Liquid embolic, Neck, Vessel
INTRODUCTION
Cerebral aneurysms are found in 45 million individuals worldwide, and approximately 30,000 patients in the United states suffer from subarachnoid hemorrhage annually.[
A new generation of liquid embolics, including PPODA-QT, may be particularly useful for difficult-to-treat aneurysms. In lieu of a porous network of coils or a metal mesh that only partially blocks flow in an aneurysm, PPODA-QT can completely fill the aneurysm volume with a mass that is both solid and stable.[
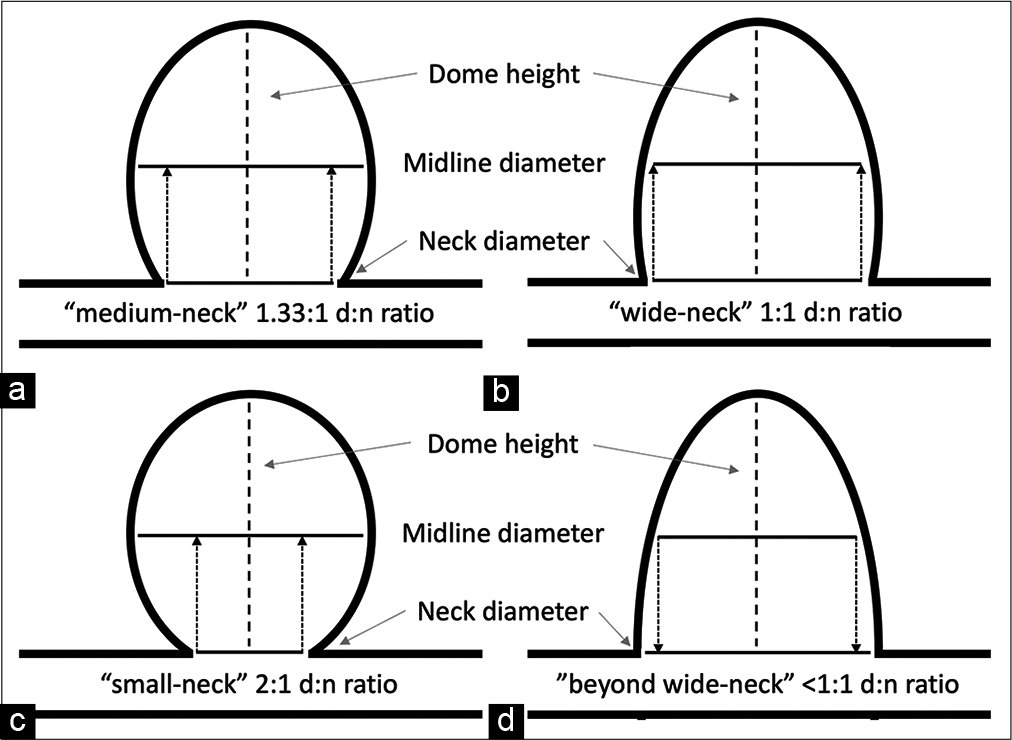
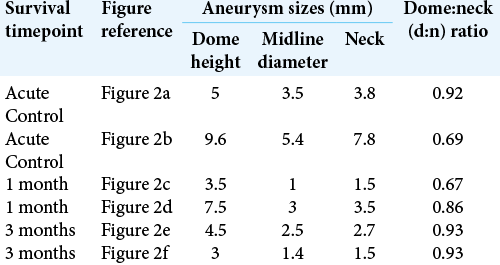
Several parameters, including aneurysm dome height and d: n ratio, are widely used to classify difficult-to-treat aneurysms. For this study, dome height is categorized as small (<5 mm height), medium (5–10 mm height), large (10–25 mm height), and giant (>25 mm height).[
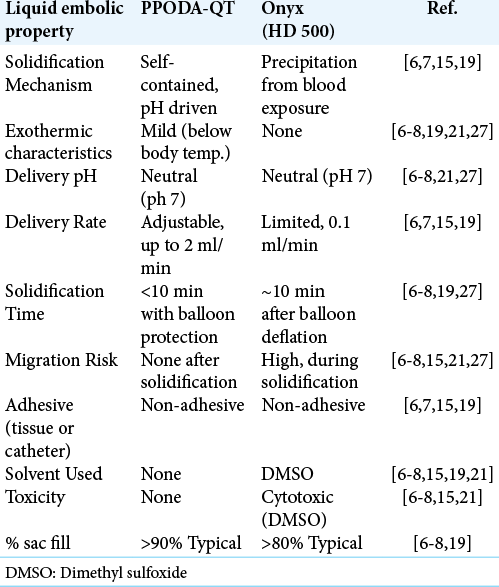
PPODA-QT is a water-based (no organic solvents), self-gelling, and non-adhesive device that exhibits superior mechanical properties and biocompatibility when compared to both metal devices and Onyx. A volume of PPODAQT, equivalent to the volume of the aneurysm dome, is delivered and solidified in a short time-frame, without exposure to blood flow, under a single balloon inflation cycle. The result is a complete and cohesive occlusion without distal embolization, cytotoxic effects, or delivery catheter adherence to the vessel wall or to the device.[
PPODA-QT is composed of liquid monomer precursors (polypropylene glycol and pentaerythritol tetrakis [3-mercapto-propionate]) delivered in a pH-titrated, injectable contrast media. When mixed, the components undergo rapid, self-contained cross-linking into a stable gel. This material exhibits a safer and faster polymerization than Onyx – which is reliant on elution of cytotoxic organic solvents into the blood stream as it slowly precipitates into a semi-solid mass while in contact with blood flow (resulting in a high risk of migration).[
Preliminary studies show that PPODA-QT consistently forms a stable gel, delivered in a single balloon inflation cycle (<10 min) without unstable material migration, particulation, or balloon-induced ischemia.[
The goal of this study was to further assess the efficacy of PPODA-QT by evaluating the tissue response following embolization of cerebral aneurysms using a well-characterized in vivo rabbit-elastase aneurysm model in preparation for a future FDA investigational device exemption (IDE) submission. In addition, the rabbit elastase aneurysm model was assessed for applicability with balloon-assisted endovascular device delivery.
MATERIALS AND METHODS
All animal study methods followed guidelines of, and were approved by, the Institutional Animal Care and Use Committees of Northern Arizona University (NAU – Flagstaff, AZ) and the Barrow Neurological Institute (BNI – Phoenix, AZ). A total of 14 New Zealand white rabbits (4-5 kg) were elastase-incubated and survived for at least 3 weeks to allow for aneurysm maturation. Eight initial rabbits were used to develop the model and assess final aneurysm dimensions through fluoroscopic imaging prior to animal terms. Six additional rabbits were enrolled to acquire aneurysm embolization survival and control data. All six rabbits underwent histological assessment. The well-established endovascular elastase incubation technique was employed to create the aneurysm models.[
Endovascular elastase incubation
The right common carotid artery (RCCA) was exposed through a one-inch incision along the jugular groove, lateral to the thymus. A 5F introducer sheath was inserted in the artery with a 6-0 suture used to ligate the artery around the sheath. A 3F Fogarty balloon catheter was introduced through the sheath and inflated at the carotid’s origin. A 0.017” ID microcatheter, (SL-10® - Stryker Neurovascular, Fremont, CA) cut to 12” long to reduce dead volume and fitted with a lure hub attachment (Nordson Medical, Minneapolis, MN), was also introduced through the sheath to deliver the elastase solution. For the endovascular technique, the elastase solution was mixed 3:1 with Isovue® 370 liquid contrast to improve fluoroscopic visualization during injection. 0.3 mL of elastase solution, containing 50 U elastase (0.15 ml of lyophilized porcine elastase dissolved in sterile PBS, titrated to a pH of 9 with 1 M NaOH, and mixed with 0.15 ml of 0.5 M CaCl2) was injected through the microcatheter.[
The effects of elastase on the vessel wall after a 3-week maturation (acute control) and the effect of PPODA-QT (survival) on the elastase aneurysms were assessed by histological examination. Histology from acute, 1-month, and 3-month rabbit models was evaluated to assess the vessel integrity and tissue response following elastase incubation and PPODA-QT embolization (n = 6).
PPODA-QT delivery technique
PPODA-QT was delivered through a dual catheter delivery technique: microcatheter delivery (Velocity® - Penumbra Inc., Alameda, CA) under balloon protection (Scepter-XC® - Microvention Inc., Aliso Viejo, CA). A 5F introducer sheath was placed in the right femoral artery of the rabbit. When inserted directly into the 5F sheath, the introducer accommodated both the 2.6F Velocity microcatheter and the 3F Scepter balloon. This technique was used because it proved difficult to consistently place 5F or larger introducer sheaths in the small rabbit femoral or Iliac arteries without risking surgical-based complications for the survival studies. Dual femoral access with 4F or smaller introducers was avoided due to the risk of post-operative blood flow disruption to both legs of a survival rabbit.
The embolization procedure began with a 1–2 min mixing step by adding liquid contrast (Conray®) at a pH of 10–11. The increase in pH initiates a mildly-exothermic reaction lasting 10 min (±30 s). The pH is self-contained in the gel, and benchtop pH testing of the PPODA-QT in a PBS solution (pH 6.5–7) showed a pH variation <2%. The resulting temperature increase is <10°C during the 10-min gelation (initiated at room temperature and remains below body temperature). Under balloon protection a PPODA-QT volume, equivalent to the aneurysm volume (determined by fluoroscopy and calculated with AngioCalc. com), was injected by hand in less than 2 min (max flow rate of 2 ml/min).[
Histology preparation
The rabbit RCCA elastase aneurysm tissues and left common carotid artery control tissues were harvested and fixed in a standard formalin solution. The samples were then placed in a tissue processor and paraffin-embedded. Tissues were sliced into 10 μm sections to provide a frontal view of the aneurysm and parent vessel. Serial sections of tissue were stained with alternating hematoxylin and eosin (H & E) and Van Gieson stains.
The H & E staining procedure followed a standard protocol. For the Van Gieson stain, slide-mounted samples were saturated with Verhoeff ’s hematoxylin, which contributed to a black appearance of elastin fibers and cell nuclei. Subsequently, the tissue was differentiated with ferric chloride, placed in the Van-Gieson counterstain to distinguish the collagen and muscle layers with red coloring, and dehydrated. All stained samples were viewed using a Zeiss Axio A1 light microscope and digitized with a Zeiss AxioCam MRc5 camera.
RESULTS
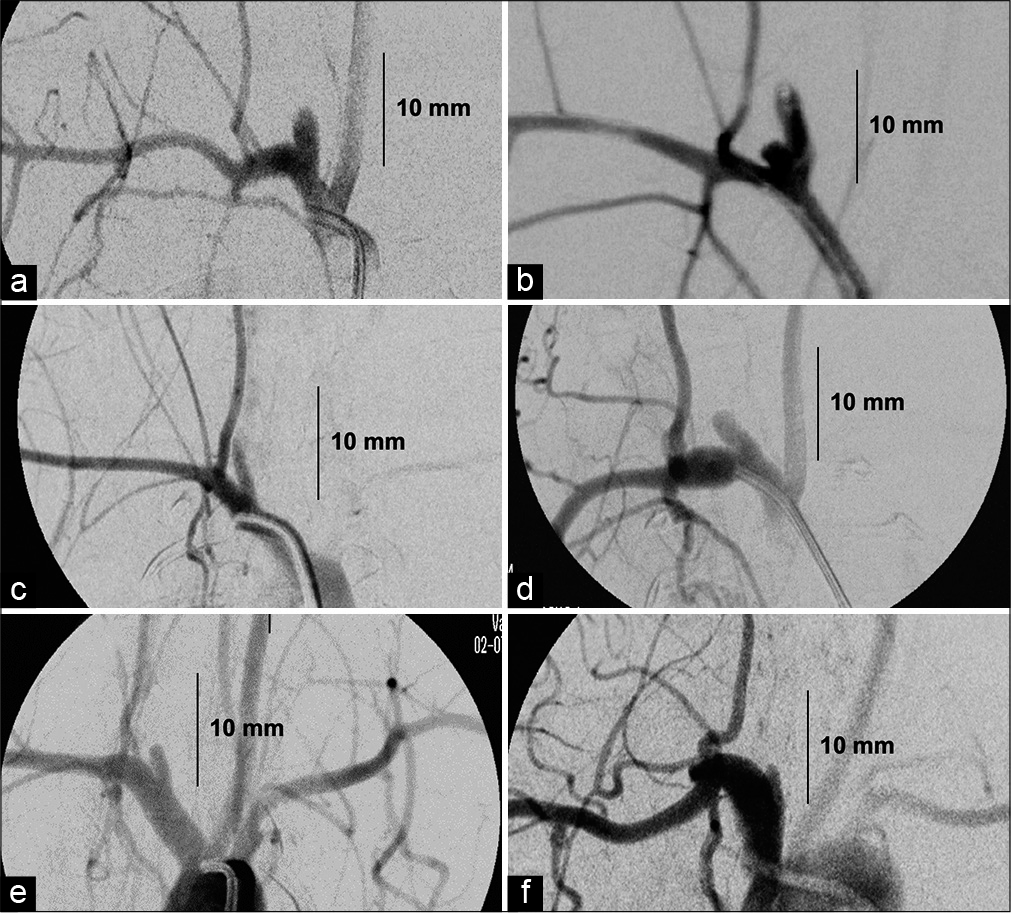
All aneurysms were created by incubating 30 mm of carotid vessel segment with elastase. However, all the aneurysms matured into small (<10 mm dome height) domes with beyond wide-necks (<1:1 d: n ratio) [
Although the aneurysms developed using this model did not match the desired larger sized (>10 mm dome height) aneurysms with medium- to wide-necks (>1.1:1 d: n ratios) intended for PPODA-QT injection [
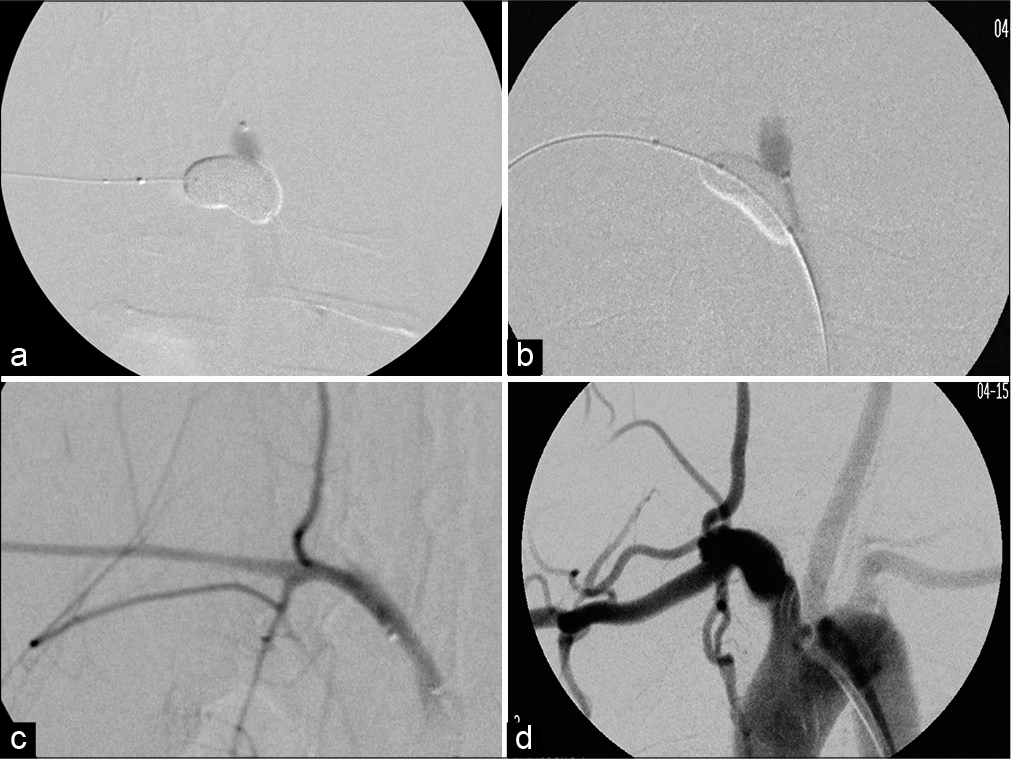
Figure 3:
Examples of PPODA-QT injection with balloon protection and post-embolization results under digital subtraction angiography (DSA): (a) is the injection of aneurysm 2a, (b) is the injection of aneurysm 2f. (c) is the post embolization result of acute aneurysm 2b and (d) is the post embolization result of 3-month survival aneurysm 2f.
Histology results
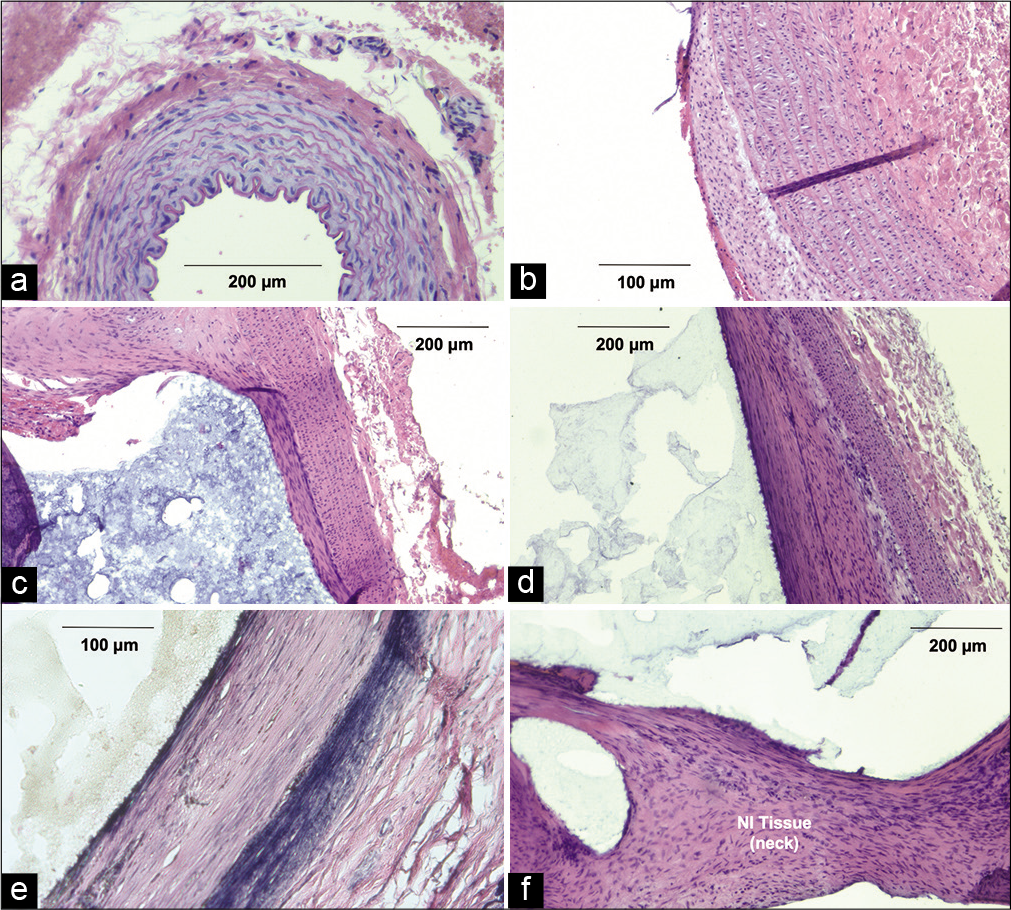
Histological data from the control tissue displayed a unicellular layer of undisrupted tunica intima, evenly distributed elastin fibers throughout the tunica media, and a loosely-organized tunica adventitia [
Figure 4:
Rabbit arterial tissue for control, acute, 1-, and 3-month timepoints. (a) Arterial cross section from control rabbit, hematoxylin and eosin (H&E) at ×100. (b) Acute aneurysm wall after elastase injection, H&E (×300). (c) Rabbit aneurysm 1 month after embolization with arterial-PPODA-QT interface, H&E (×100). (d) Aneurysm wall 3 months after embolization with arterial-PPODA-QT interface (×100). (e) Aneurysm wall 3 months after embolization with arterial-PPODA-QT interface, Van Gieson (×100). (f) Neo-intimal aneurysm neck with displaced PPODA-QT artifact, H&E (×300).
The cross-section of an H & E-stained acute control aneurysm, which matured for 3 weeks post-elastase incubation, exhibited no apparent tissue damage or immune response [
The 1- and 3-month aneurysm histology [
The Van Gieson stain from the same 3-month tissue demonstrated a reorganization of the vessel wall. The more distal layer showed an organized, dense, and linear pattern of elastin while the proximal layer (at the aneurysm lumen) was less uniform and replaced by the reorganized smooth muscle layer [
DISCUSSION
Aneurysm size is a direct correlation to the risk of aneurysm rupture, and therefore large aneurysms are likely candidates for treatment. However, these larger aneurysms with medium- and wide-necks also suffer from an increased frequency of incomplete occlusion in the short-term and high recanalization rates in the long-term following endovascular treatment.[
In recent years, rabbit elastase models have grown in popularity due to physiological similarities to humans, availability, and economic feasibility as an animal model. Although rabbit vessels do not scale to the human vasculature to the extent of canines and swine, they have a similar clotting cascade and healing process when compared to humans. The elastase aneurysm creation procedure has also been touted as less surgically invasive, potentially resulting in a more effective differentiation of the immune responses from the aneurysm surgery and the subsequent device implantation.[
In general, the response of arterial tissue to foreign bodies such as biomaterial devices is regulated by the device’s surface properties. The reaction occurs within the first 2–4 weeks after implantation and continues for the entire duration of the tissue-device interface.[
Although metal coils remain the “gold standard” for treatment of medium aneurysms with small and medium d: n ratios and neck diameters <4 mm, these coils (including the newer curtain mesh design, i.e., Medina®, Medtronic) fill <30% of the aneurysm volume.[
Flow Diverters, such as the Pipeline® embolization device, redirect blood flow away from the aneurysm. However, these devices can be difficult to navigate, deploy, and properly expand in tortuous vessels and can increase complication rates (30–40% in some series).[
Flow disrupters consist of an intrasaccular metal frame that expands in the aneurysm, thereby reducing the metal surface exposure at the parent vessel in an attempt to reduce thrombogenic effects.[
Coils, WEB devices, and flow diverters are all composed of metal alloys, which have limited compatibility with blood and can promote local or downstream thrombus. These devices also exhibit inconsistent healing responses, especially in larger aneurysms with medium-to-wide-neck d: n ratios, resulting in high aneurysm recanalization rates.[
Limitations of previous liquid embolics
Polymer-based devices, such as liquid embolics, have mechanical properties that closely approximate natural tissue, can completely fill varied aneurysm morphologies, and exhibit high biocompatibility compared to their metal counterparts.
However, liquid embolics for cerebral aneurysm embolization have not been widely accepted, due in large part to the limitations observed in the only available aneurysm treatment material: Onyx HD-500® [
CONCLUSION
The results of the present study suggest that PPODA-QT can be successfully delivered to wider-neck aneurysms, leading to aneurysm tissue reorganization and stabilization that facilitates continuous healing at the aneurysm neck. Ultimately, such a result may reduce recanalization and rupture of larger aneurysms. Because PPODA-QT treatment currently targets larger aneurysms than those created by the rabbit elastase model, further evaluation of more complex aneurysm models with a statistically significant number of subjects is the next step. Surgical anastomosis canine models provide more flexibility for creating larger aneurysms with consistent d: n ratios while also exhibiting a healing response similar to the human condition. Such an alternative aneurysm model will be investigated to further verify the biocompatibility results of PPODA-QT reported in this study.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
This work was supported by the National Institute of Health STTR, a sponsored research agreement - grant #1R41NS097069.
Conflicts of interest
Mark C. Preul MD, Andrew F. Ducruet MD, and Timothy A. Becker PhD have financial interest in Aneuvas Technologies, Inc. (ATI) which now manufactures a version of PPODA-QT as NeuroCURE®. Dr. Ducruet also has formal consulting roles with Stryker, Penumbra, Cerenovus, Medtronic, and Koswire. The Barrow Neurological Institute and Northern Arizona University have no commercial collaboration with ATI in the production, distribution, or marketing of NeuroCURE®.
Acknowledgment
We thank the veterinary staffs of the hospital and university for their expert technical assistance and animal care. We are grateful to Aubrey Funke and the university’s Imaging and Histology Core Facility (IHCF) for helping prepare and stain the tissue samples.
References
1. Altes TA, Cloft HJ, Short JG, Degast A, Do HM, Helm GA. Creation of saccular aneurysms in the rabbit: A model suitable for testing endovascular devices. Am J Roentgenol. 2000. 174: 349-54
2. Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008. 20: 86-100
3. Avery MB, Alaqeel A, Bromley AB, Chen YX, Wong JH, Eesa M. A refined experimental model of fusiform aneurysms in a rabbit carotid artery. J Neurosurg. 2018. 131: 88-95
4. Becker TA, Preul MC, Bichard WD, Kipke DR, McDougall CG. Preliminary investigation of calcium alginate gel as a biocompatible material for endovascular aneurysm embolization in vivo. Neurosurgery. 2007. 60: 1119-27
5. Bouzeghrane F, Naggara O, Kallmes DF, Berenstein A, Raymond J. In vivo experimental intracranial aneurysm models: A systematic review. Am J Neuroradiol. 2010. 31: 418-23
6. Brennecka C, Preul M, Becker T, Vernon B. In vivo embolization of lateral wall aneurysms in canines using the liquid-to-solid gelling PPODA-QT polymer system: 6-month pilot study. J Neurosurg. 2013. 119: 228-38
7. Brennecka CR, Preul MC, Bichard WD, Vernon BL. In vivo experimental aneurysm embolization in a swine model with a liquid-to-solid gelling polymer system: Initial biocompatibility and delivery strategy analysis. World Neurosurg. 2012. 78: 469-80
8. Brennecka CR, Preul MC, Vernon BL. In vitro delivery, cytotoxicity, swelling, and degradation behavior of a liquid-to-solid gelling polymer system for cerebral aneurysm embolization. J Biomed Mater Res B Appl Biomater. 2012. 100: 1298-309
9. Brinjikji W, Cloft HJ, Kallmes DF. Difficult aneurysms for endovascular treatment: Overwide or undertall?. Am J Neuroradiol. 2009. 30: 1513-7
10. Burrows AM, Cloft H, Kallmes DF, Lanzino G. Periprocedural and mid-term technical and clinical events after flow diversion for intracranial aneurysms. J Neurointerv Surg. 2015. 7: 646-51
11. Ding YH, Dai D, Schroeder D, Kadirvel R, Kallmes DF. Experimental testing of the dual-layer Woven EndoBridge device using an elastase-induced aneurysm model in rabbits. Interv Neuroradiol. 2016. 22: 299-303
12. Fiorella D, Arthur A, Byrne J, Pierot L, Molyneux A, Duckwiler G. Interobserver variability in the assessment of aneurysm occlusion with the WEB aneurysm embolization system. J Neurointerv Surg. 2015. 7: 591-5
13. Hayakawa M, Murayama Y, Duckwiler GR, Gobin YP, Guglielmi G, Viñuela F. Natural history of the neck remnant of a cerebral aneurysm treated with the Guglielmi detachable coil system. J Neurosurg. 2000. 93: 561-8
14. Hoh BL, Rabinov JD, Pryor JC, Ogilvy CS. A modified technique for using elastase to create saccular aneurysms in animals that histologically and hemodynamically resemble aneurysms in human. Acta Neurochir (Wien). 2004. 146: 705-11
15. Jordan O, Doelker E, Rüfenacht DA. Biomaterials used in injectable implants (liquid embolics) for percutaneous filling of vascular spaces. Cardiovasc Intervent Radiol. 2005. 28: 561-9
16. Kurre W, Berkefeld J. Materials and techniques for coiling of cerebral aneurysms: How much scientific evidence do we have?. Neuroradiology. 2008. 50: 909-27
17. McLemore R, Preul MC, Vernon BL. Controlling delivery properties of a waterborne, in situforming biomaterial. J Biomed Mater Res B Appl Biomater. 2006. 79: 398-410
18. Mine B, Pierot L, Lubicz B. Intrasaccular flow-diversion for treatment of intracranial aneurysms: The Woven EndoBridge. Expert Rev Med Devices. 2014. 11: 315-25
19. Molyneux AJ, Cekirge S, Saatci I, Gál G. Cerebral aneurysm multicenter european onyx (CAMEO) trial: Results of a prospective observational study in 20 European centers. Am J Neuroradiol. 2004. 25: 39-51
20. Norbash AM, Singer RJ. Videographic assessment of the embolic characteristics of three polymeric compounds: Ethylene vinyl alcohol, cellulose acetate, and liquid urethane. Am J Neuroradiol. 2001. 22: 334-40
21. Ozdol C, Turk CC, Hazer DB, Yildirim AE, Arat A, Atilla P. Comparison of the toxicities of ethylene vinyl alcohol copolymer (EVOH) preparations, dimethyl sulphoxide and N-butyl 2-cyanoacrylate on cerebral parenchyma in an experimental rabbit model. Turk Neurosurg. 2015. 25: 446-52
22. Piske RL, Kanashiro LH, Paschoal E, Agner C, Lima SS, Aguiar PH. Evaluation of onyx hd-500 embolic system in the treatment of 84 wide-neck intracranial aneurysms. Neurosurgery. 2009. 64: E865-75
23. Pop R, Mertz L, Ilyes A, Mihoc D, Richter JS, Manisor M. Beam hardening artifacts of liquid embolic agents: Comparison between squid and onyx. J Neurointerv Surg. 2019. 11: 706-9
24. Ries T, Groden C. Endovascular treatment of intracranial aneurysms: Long-term stability, risk factors for recurrences, retreatment and follow-up. Klin Neuroradiol. 2009. 19: 62-72
25. Riley CM, McLemore R, Preul MC, Vernon BL. Gelling process differences in reverse emulsion, in situ gelling polymeric materials for intracranial aneurysm embolization, formulated with injectable contrast agents. J Biomed Mater Res B Appl Biomater. 2011. 96: 47-56
26. Sluzewski M, Menovsky T, van Rooij WJ, Wijnalda D. Coiling of very large or giant cerebral aneurysms: Long-term clinical and serial angiographic results. AJNR Am J Neuroradiol. 2003. 24: 257-62
27. Struffert T, Roth C, Romeike B, Grunwald IO, Reith W. Onyx in an experimental aneurysm model: Histological and angiographic results. J Neurosurg. 2008. 109: 77-82
28. Tsumoto T, Song JK, Niimi Y, Berenstein A. Interval change in size of venous pouch canine bifurcation aneurysms over a 10-month period. Am J Neuroradiol. 2008. 29: 1067-70
29. van Rooij WJ, Sluzewski M. Coiling of very large and giant basilar tip aneurysms: Midterm clinical and angiographic results. AJNR Am J Neuroradiol. 2007. 28: 1405-8
30. Wiebers DO, Whisnant JP, Huston J, Meissner I, Brown RD, Piepgras DG. Unruptured intracranial aneurysms: Natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003. 12: 103-10
31. Youn SO, Lee JI, Ko JK, Lee TH, Choi CH. Endovascular treatment of wide-necked intracranial aneurysms using balloon-assisted technique with hyperform balloon. J Korean Neurosurg Soc. 2010. 48: 207-12