- Department of Neurosurgery, Istituto di Ricovero e Cura a Carattere Scientifico Ospedale San Raffaele, Milan, Italy
- Department of Neurosurgery, Ospedale San Raffaele, Milan, Italy
Correspondence Address:
Carlo Mandelli, Department of Neurosurgery, Istituto di Ricovero e Cura a Carattere Scientifico Ospedale San Raffaele, Milan, Italy.
DOI:10.25259/SNI_173_2025
Copyright: © 2025 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Carlo Mandelli1, Cinzia Mura2, Luigi Albano1, Pietro Mortini1. Brachial plexus exposure in 19 post-traumatic patients: A morphometric anatomic analysis. 30-May-2025;16:203
How to cite this URL: Carlo Mandelli1, Cinzia Mura2, Luigi Albano1, Pietro Mortini1. Brachial plexus exposure in 19 post-traumatic patients: A morphometric anatomic analysis. 30-May-2025;16:203. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13600
Abstract
Background: Traumatic brachial plexus injuries result in severe functional impairment, significantly affecting patients’ quality of life. This study aims to evaluate the clinical outcomes of surgical interventions in 19 patients with brachial plexus injuries, emphasizing the effectiveness of linear incision techniques. Our surgical objectives included restoring shoulder control, elbow flexion, and elbow extension, alongside optimizing sensory recovery.
Methods: We performed morphometric analyses to standardize surgical approaches, utilizing topographic linear incisions for enhanced exposure and precision. This technique ensures critical advantages: short incisions with excellent nerve visualization, identification of consistent anatomical landmarks for safe dissection even amidst scar tissue, and facilitation of multiple neurotizations through single incisions. This approach is inspired by and builds on the principles outlined by Bertelli and Ghizoni, whose work highlighted the importance of precise anatomical dissection and efficient nerve exposure.
Results: We collected all morphometric measurements to standardize surgical approaches. Key findings demonstrated that linear incisions improved surgical efficiency and facilitated tension-free nerve anastomoses without the need for grafts in most cases.
Conclusion: Linear incision techniques, supported by the foundational principles established by Bertelli and Ghizoni, provide significant clinical benefits in brachial plexus surgery. This approach enhances both surgical precision and patient recovery while supporting the achievement of critical neurofunctional objectives.
Keywords: Brachial plexus injuries, Nerve grafting, Nerve transfer, Neurotization
INTRODUCTION
Traumatic brachial plexus injuries are increasingly frequent, causing significant physical and social disability due to the loss of upper limb strength, sensitivity, and hand function. These injuries are often associated with penetrating trauma, falls, or motor vehicle accidents, particularly involving high-speed motorcycles, and most commonly affect men aged 16–25 years.[
Management requires a multidisciplinary approach involving specialists in rehabilitation, neurology, physical and occupational therapy, and peripheral nerve surgery. The approach depends on the injury’s mechanism, location, and whether it is “open” or “closed.” Early surgical intervention is indicated for open injuries caused by sharp or penetrating trauma that transect nerves. For blunt or stretch injuries, electrodiagnostic studies are recommended at 6 weeks and 3 months, with surgical planning initiated if no improvement is observed.
Advances in peripheral nerve surgery have improved outcomes, but treatment combines evidence-based principles, feasibility, and surgeon expertise, with the primary goal being to restore motor and sensory neurological function.
Patients were considered eligible for surgical treatment if they met the following criteria:
Patients with avulsion injuries; Patients with extensive neuromas; Patients with axonotmesis damage without recovery after the observation period; Patients with a combination of the above patterns.
We performed a morphometric analysis on 19 patients who met the criteria for surgical candidacy. The surgical techniques applied were based on descriptions in the literature, with reference to the works of Bertelli and Ghizoni.[
Short incisions with excellent exposure of the nervous elements; Identification of fixed anatomical landmarks that ensure nerve isolation even in the presence of scar tissue; Selection of the most suitable donor nerves; Combination of multiple neurotizations with a single incision; Ipsilateral and synergistic neurotizations; Distal harvesting of donor nerves and nerve sutures as close as possible to the muscle targeted for reinnervation; Anastomoses performed without tension and grafts in most neurotizations; Facilitation and acceleration of surgical approaches.
MATERIALS AND METHODS
The study was conducted by obtaining morphometric measurements during the various surgical steps. During each step, a measurement table was completed for each approach.
The aims of our brachial plexus surgery on 19 patients included:
Shoulder control (abduction, external rotation, and scapular stabilization) Elbow flexion Elbow extension and restoration of sensation in the median nerve distribution whenever possible, or in cases of multilevel avulsion injuries associated with neuropathic pain.[
All procedures were performed with neurophysiological monitoring.
Shoulder control
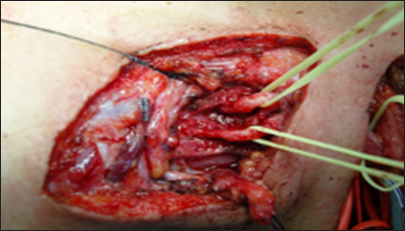
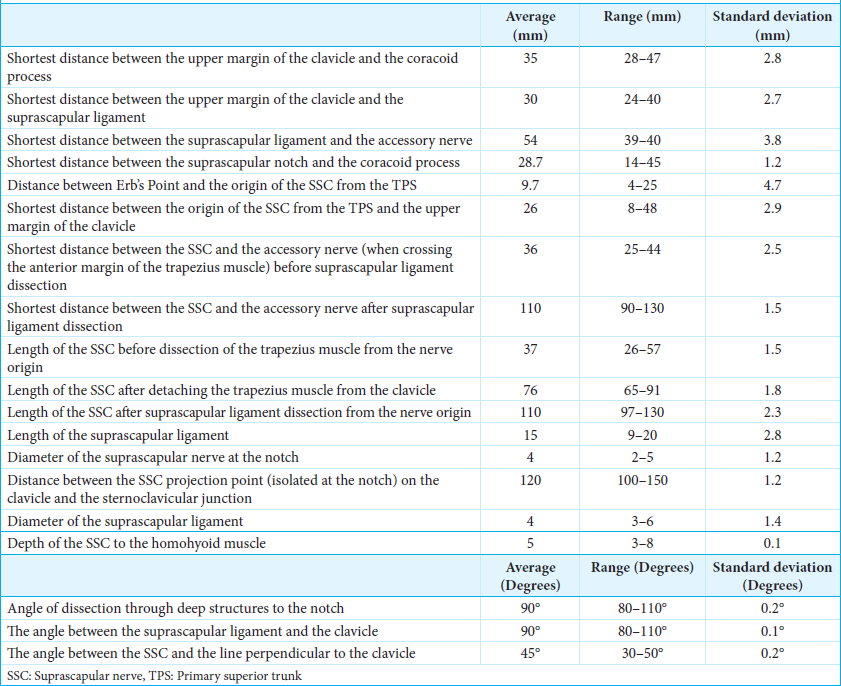
In cases of non-avulsion injuries, the first step of surgery involves achieving shoulder control, specifically, abduction, external rotation, and scapular stabilization, by exposing the C5 and C6 nerve roots. The procedure also includes exposing the XI cranial nerve and the suprascapular nerve, as outlined below.
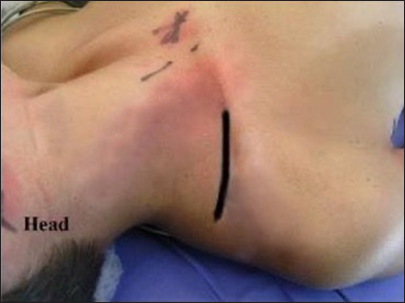
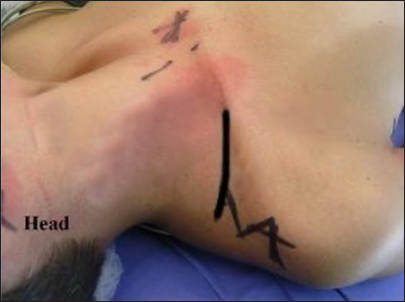
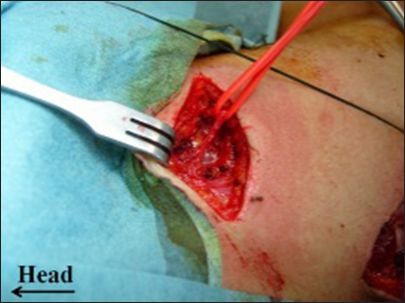
The initial incision described is a linear oblique one, allowing for root exposure slightly lateral to Chassaignac’s tubercle [
The second incision is an oblique supraclavicular one, starting at the junction between the medial and middle thirds of the clavicle[
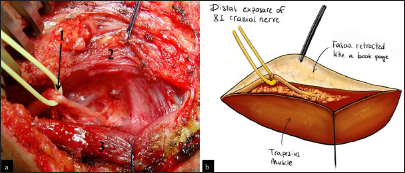
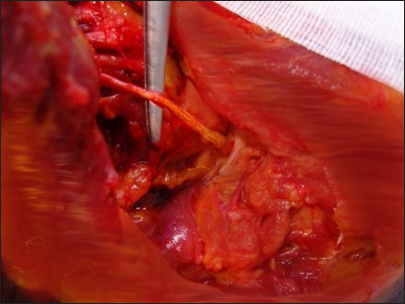
This posterior portion of the incision, located at the anterior edge of the trapezius muscle, facilitates exposure of the XI cranial nerve using the “book-opening” technique described by Bertelli and Ghizoni. The subcutaneous tissue is dissected, and the XI cranial nerve is isolated at the anterior border of the trapezius muscle, approximately 4 cm from the clavicle, with the aid of an intraoperative microscope. Specifically, the spinal accessory nerve is identified within the fat between the trapezius muscle and its fascia, which is retracted like a book page, in an avascular plane, as described by Bertelli and Ghizoni [
On the other hand, the anterior portion of the same second incision allows access to the omohyoid muscle, which can be retracted anteriorly and serves as a landmark for exposing the suprascapular nerve, originating from the superior trunk of the brachial plexus. The nerve is identified at a deeper plane beneath the omohyoid muscle [
Figure 5:
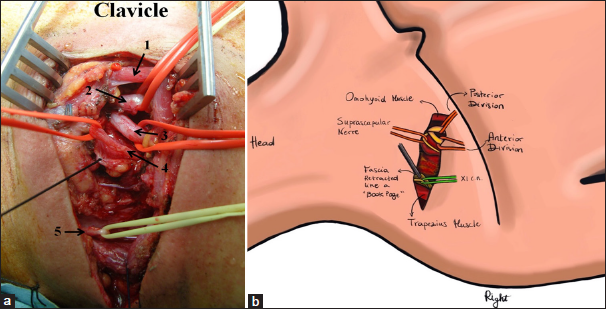
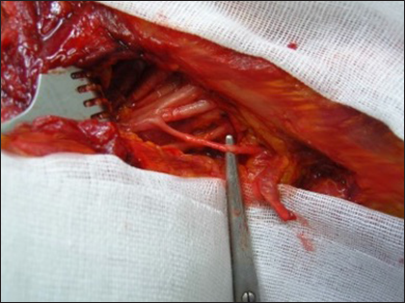
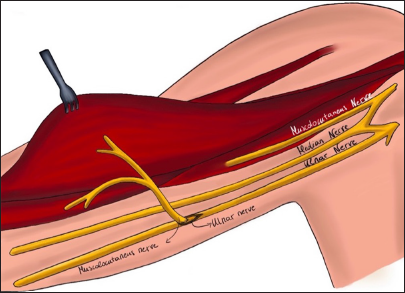
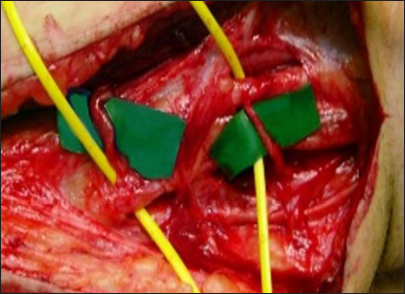
(a) Linear incision in the supraclavicular region. It begins at the junction between the medial third and the lateral two-thirds of the clavicle and extends toward the trapezius muscle. In a disto-proximal direction, the following anatomical structures are visible: the retracted omohyoid muscle (1), the posterior division (2), the anterior division (3), the suprascapular nerve (4), and the spinal accessory nerve (XI cranial nerve, 5) - right side. (b) Anatomic illustration.
In summary, moving in a distal-to-proximal direction, the following anatomical structures are identified: the retracted omohyoid muscle, the posterior division, the anterior division, the suprascapular nerve, and the spinal accessory nerve at the posterior aspect of the second incision [
The third supraclavicular incision is linear, with a lateral extension toward the acromion in a zigzag pattern [
The deep fascia over the trapezius muscle is dissected, and the muscle is detached from the clavicle and acromion. The coracoid process, located beneath the clavicle within the fat tissue, is identified by palpation. The final step involves exposing the ligament under magnification [
In addition, we routinely expose the motor branch of the platysma as described by Bertelli[
Elbow flexion
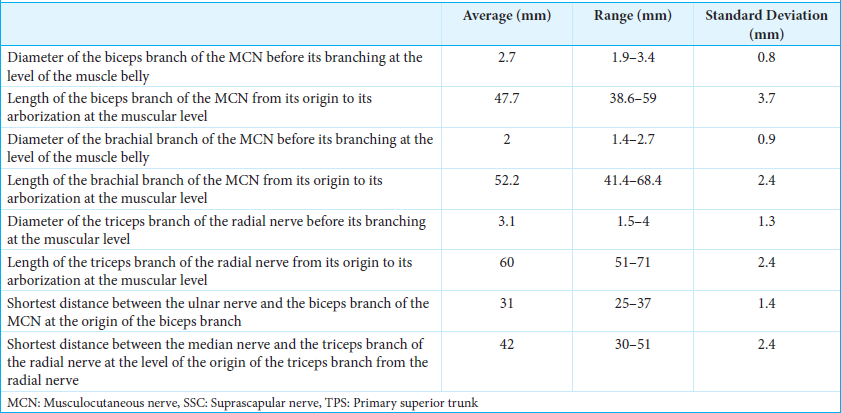
Restoration of elbow flexion, in cases where the function of the inferior brachial plexus is preserved, is performed using the classic Oberlin neurotization described in 1994, with the partial transfer of the ulnar nerve to the biceps motor branch of the musculocutaneous nerve (MCN) (7,15,21) [
In cases where triceps function is preserved, we always perform a double Oberlin technique with the transfer of double fascicles from the ulnar and median nerves to the MCN branches to the brachialis and biceps. All 9-0 nonresorbable sutures should not be under tension during both arm flexion and extension.
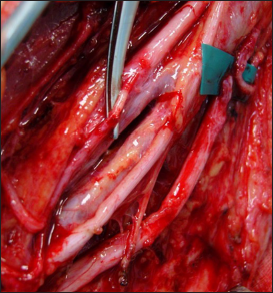
An 8 cm long incision was made on the medial aspect of the arm over the brachial vessels, beginning 4 cm distal to the lateral border of the pectoralis major. The fascia over the biceps was dissected from the coracobrachialis muscle, and the motor branch of the biceps was identified. The motor branch was traced as far proximally as possible into the MCN and was then divided. A second fascial incision was performed posterior to the intermuscular septum, and the ulnar nerve was exposed for 3 cm. A longitudinal epineurotomy was made on the anterolateral region of the ulnar nerve, which was coapted to the biceps motor branch.[
Elbow extension
In patients with elbow extension impairment, we use the surgical exposure for the Oberlin neurotization to select two fascicles of the median nerve to the flexor carpi radialis (FCR) that are coapted to the motor branch of the triceps long head (recipient nerve).[
The triceps long head motor branch is traced up to its arborization inside the muscle and then cut at its origin from the radial nerve and transferred to the median nerve fascicles, performing a 9-0 nylon suture [
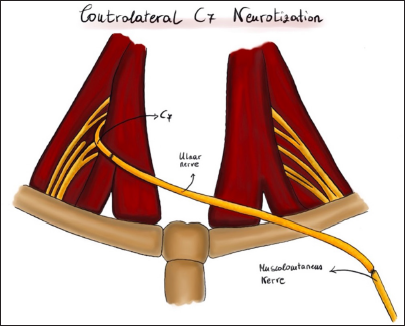
Contralateral C7
In cases of young patients with complete avulsion of the brachial plexus and the impossibility of using the intercostal nerves, we connect the contralateral half C7 root to the MCN using the vascularized homolateral ulnar nerve [
This C7 component can be transversally sectioned under the intraoperative microscope in its apical posterior segment (in our patients, no more than 50% of the root was sectioned). The ulnar nerve at the traumatic site is entirely exposed until the wrist and segmented at this level. The temperature of the operating room should not be too low to avoid vasoconstriction of the vasa nervorum. The superior ulnar collateral artery is preserved [
RESULTS
This study analyzed 19 patients who underwent surgical treatment for brachial plexus injuries based on the degree and topographic level of brachial plexus damage, with outcomes assessed according to key functional objectives: shoulder control, elbow flexion, and elbow extension. The detailed morphometric measurements and surgical techniques applied were critical for optimizing nerve exposure, mobilization, and coaptation, ensuring tension-free and anatomically precise repairs [
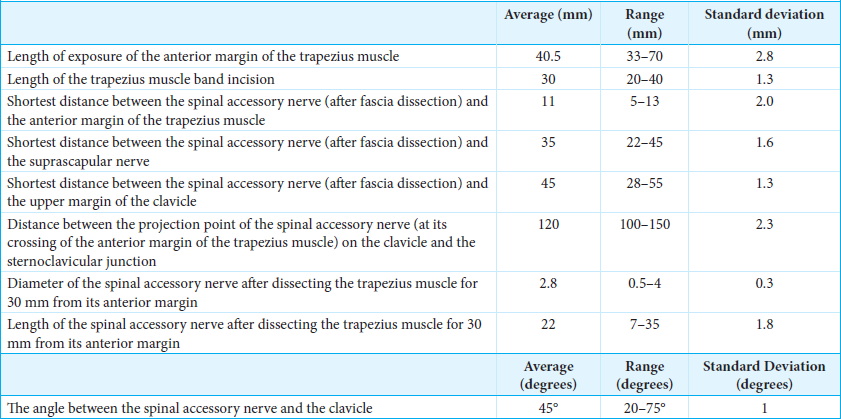
The XI cranial nerve and the suprascapular nerve were exposed through an oblique supraclavicular incision, starting at the junction between the medial and middle thirds of the clavicle [
The same surgical incision allows for the identification of the suprascapular nerve [
The final step was the exposure and sectioning of the ligament. The length of the suprascapular ligament was 15 mm (range 9–20 mm). The diameter of the suprascapular nerve at the notch was 4 mm (range 2-5 mm). The depth of the suprascapular nerve from the omohyoid muscle was 5 mm (range 3–8 mm). After the ligament was transected, it was possible to mobilize the suprascapular nerve until the notch, obtaining nerve exposure of approximately 11 cm (range 97–130 mm) in length from its origin. The angle of dissection through deep structures to the notch was 90° (range 80–110°), and the angle between the suprascapular ligament and the clavicle was 90° (range 80–110°) [
The platysma motor branch was exposed with a 3 cm incision located one finger below the mandible [
The Oberlin procedure, aimed at restoring elbow flexion, involves transferring a fascicle from the ulnar nerve to the musculocutaneous branch to the biceps. An 8 cm long incision was made on the medial aspect of the arm over the brachial vessels, beginning 4 cm distal to the lateral border of the pectoralis major [
To restore elbow extension, the same surgical exposure for the Oberlin neurotization was used to select two fascicles of the median nerve to the FCR, which were coapted to the triceps head motor branch. The shortest distance between the median nerve and the triceps branch of the radial nerve was 42 mm (range 30–51 mm). The diameter of the triceps branch of the radial nerve before its branching at the muscular level was 3.1 mm (range 1.5–4 mm). The length of the triceps branch of the radial nerve from its origin to its arborization at the muscular level was 60 mm (range 51–71 mm) [
DISCUSSION
Different techniques and experiences of brachial plexus surgical reconstruction are described in the medical literature.[
According to Bertelli and Ghizoni’s techniques, upper brachial plexus avulsions are treated by restoring the suprascapular nerve function using the XI cranial nerve harvested at the anterior border of the trapezius muscle.[
Opening the trapezius fascia like a book page [
The integrity of the C5 and C6 roots is evaluated using our specific magnetic resonance imaging protocol before their grafting and connection with the anterior and posterior divisions.[
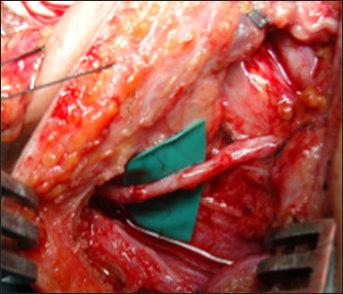
As a collateral reinforcing neurotization, we typically add a connection between the platysma motor branch and the medial pectoral nerve or the anterior division, interposing a 10 cm sural nerve graft [
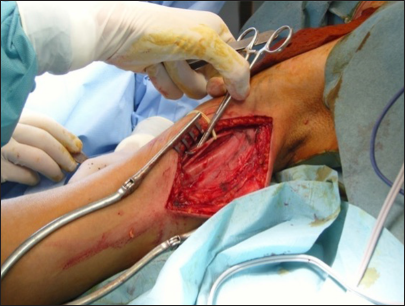
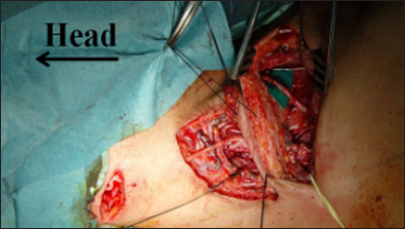
Figure 22:
Final view of the three linear approaches that allowed us to perform the following connections: XI cranial nerve versus suprascapular nerve direct neurotization, platysma motor branch versus anterior division neurotization through the interposition of the sural nerve, sural grafting between C5 and the posterior division, and another graft between C6 and the anterior division – right side.
In cases of selected superior brachial plexus injury, we restore elbow flexion using Oberlin neurotization as described in the medical literature [
In our experience, the intercostal-to-MCN transfer should be used as a second choice to restore elbow flexion, due to the time-consuming and challenging nature of the surgery. Intercostal nerves are very thin, and the risk of damage to the anastomosis should be considered [
In young patients affected by total avulsion or damage to the entire brachial plexus with no intercostal nerves available (e.g., chest trauma, pulmonary pathologies),[
The reasons for non-recovery are related to biological aspects, such as a lower chance of improvement in older patients due to reduced regenerative capacity and a decreased ability to follow rehabilitation. Furthermore, patients with complete paralysis of the arm and advanced muscular atrophy showed a reduced likelihood of achieving functional results. On the other hand, some patients failed to recover due to surgical factors, such as the possible disruption of nerve-to-nerve coaptation.[
CONCLUSION
This study demonstrates the effectiveness of advanced surgical techniques for traumatic brachial plexus injuries, emphasizing precise nerve coaptation and strategic donor nerve selection. Key procedures such as XI cranial nerve-to-suprascapular nerve neurotization and Oberlin transfer yielded promising results in restoring shoulder and elbow function. The incorporation of intraoperative morphometric analysis, including nerve length and diameter measurements, enabled accurate, tension-free coaptation, significantly enhancing functional outcomes. Our findings confirm that timely intervention, combined with careful surgical planning guided by morphometric data and the use of topographically localized linear incisions and direct neurotizations, can achieve substantial functional recovery and improve patients’ quality of life.
Ethical approval:
The Institutional Review Board approval was not required because this is a retrospective study .. The study adhered to the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Declaration of patient consent:
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship:
Nil.
Conflicts of interest:
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation:
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
Acknowledgments:
CM is the primary author of this article. He collected morphometric measurements and wrote the manuscript. CM(Mura) contributed to the writing and created the anatomical illustrations. LA assisted in the article’s revision. PM supervised and approved the final version.
References
1. Bertelli JA. Platysma motor branch transfer in brachial plexus repair: Report of the first case. J. Brachial Plex Periph Nerve Inj. 2007. 2: 12
2. Bertelli JA, Ghizoni MF. Controlateral motor rootlets and ipsilateral nerve transfers in brachial plexus reconstruction. J Neurosurg. 2004. 101: 770-8
3. Bertelli JA, Ghizoni MF. Concepts of nerve regeneration and repair applied to brachial plexus reconstruction. Microsurgery. 2006. 26: 230-44
4. Bertelli JA, Ghizoni MF. Improved technique for harvesting the accessory nerve for transfer in brachial plexus injuries. Neurosurgery. 2006. 58: 366-70
5. Bertelli JA, Ghizoni MF. Long thoracic nerve: Anatomy and functional assessment. J Bone Joint Surg Am. 2005. 87: 993-8
6. Bertelli JA, Ghizoni MF. Nerve repair by end-to-side coaptation or fascicular transfer: A clinical study. J Reconstr Microsurg. 2003. 19: 313-8
7. Bertelli JA, Ghizoni MF. Reconstruction of C5 and C6 brachial plexus avulsion injury by multiple nerve transfers: Spinal accessory to suprascapular, ulnar fascicles to biceps branch, and triceps long or lateral head branch to axillary nerve. J Hand Surg. 2004. 29: 131-9
8. Bertelli JA, Ghizoni MF. Transfer of the accessory nerve to the suprascapular nerve in brachial plexus reconstruction. J Hand Surg. 2007. 32: 989-98
9. Bertelli JA, Kechele PR, Santos MA, Duarte H, Ghizoni MF. Axillary nerve repair by triceps motor branch transfer through an axillary access: Anatomical basis and clinical results. J Neurosurg. 2007. 107: 370-7
10. Bertelli JA, Kechele PR, Santos MA, Duarte H, Ghizoni MF. Triceps motor nerve branches as a donor or receiver in nerve transfers. Neurosurgery. 2007. 61: 333-8 discussion 338-9
11. Bowen BC, Pattany PM, Lavi ES, Maravilla KR. The brachial plexus: Normal anatomy, pathology, and MR imaging. Neuroimaging Clin N Am. 2004. 14: 59-85
12. Brophy RH, Wolfe SW. Planning brachial plexus surgery: Treatment options and priorities. Hand Clin. 2007. 21: 47-54
13. Chalidapong P, Sananpanich K, Kraisarin J, Bumroongkit C. Pulmonary and Biceps function after intercostal and phrenic nerve transfer for brachial plexus injuries. J Hand Surg (Br). 2004. 1: 8-11
14. Chuang DCC. Nerve transfers in adult brachial plexus injuries: My methods. Hand Clin. 2005. 21: 71-82
15. Ferraresi S, Garozzo D, Buffetti P. Reinnervation of the biceps in C5-7 brachial plexus avulsion injuries: Results after distal bypass surgery. Neurosurg Focus. 2004. 16: 1-5
16. Gerevini S, Mandelli C, Cadioli M, Scotti G. Diagnostic value and surgical implications of magnetic resonance imaging in the management of adult patients with brachial plexus pathologies. Surg Radiol Anat. 2008. 30: 91-101
17. Harper CM. Preoperative and intraoperative electrophysiologic assessment of brachial plexus injuries. Hand Clin. 2005. 21: 39-46
18. Liverneaux PA, Diaz LC, Beaulieu JY, Durand S, Oberlin C. Preliminary results of double nerve transfer to restore elbow flexion in upper type brachial plexus palsies. Plast Reconstr Surg. 2006. 117: 915-9
19. Malessy JA, Thomeer RT. Evaluation of intercostal to musculocutaneous nerve transfer in reconstructive brachial plexus surgery. J Neurosurg. 1998. 88: 266-71
20. Moran SL, Steinmann SP, Shin AY. Adult brachial plexus injuries: Mechanism, patterns of injury, and physical diagnosis. Hand Clin. 2005. 21: 13-24
21. Oberlin C, Beal D, Leechavengvongs S. Nerve transfer to biceps muscle using a part of ulnar nerve for C5-C6 avulsion of the brachial plexus: Anatomical study and report of four cases. J Hand Surg (Am). 1994. 19: 232-7
22. Songcharoen P, Wongtrakul S, Spinner RJ. Brachial plexus injuries in the adult. Nerve transfers: The Siriraj Hospital experience. Hand Clin. 2005. 21: 83-9
23. Spinner RJ, Shin AY, Bishop AT. Update on brachial plexus surgery in adults. Curr Opin Orthop. 2004. 15: 203-14
24. Waikakul S, Orapin S, Vanadurongwan V. Clinical results of controlateral C7 root neurotization to median nerve in brachial plexus injuries with total root avulsions. J Hand Surg. 1999. 24: 556-60